Beyond natural pathway: an artificial pathway for malonyl-CoA biosynthesis
Published in Bioengineering & Biotechnology, Chemistry, and Microbiology

Malonyl-CoA plays a crucial role in biosynthesis as a building block for the production of various natural products including polyketides, flavonoids, coumarins, and stilbenes (Fig. 1). While acetyl-CoA carboxylation is the canonical route for endogenous malonyl-CoA formation found in nature, this pathway suffers from slow kinetics, carbon and energy inefficiencies, tight regulations, complicated architecture and severe cellular toxicity, which limit flux towards malonyl-CoA and become a decisive bottleneck limiting the biosynthesis of all malonyl-CoA-derived products (MDPs). Hence, traditional metabolic engineering efforts, such as overexpression and/or modification of natural pathway, show very limited positive or even negative effect on the synthesis of malonyl-CoA and MDPs.
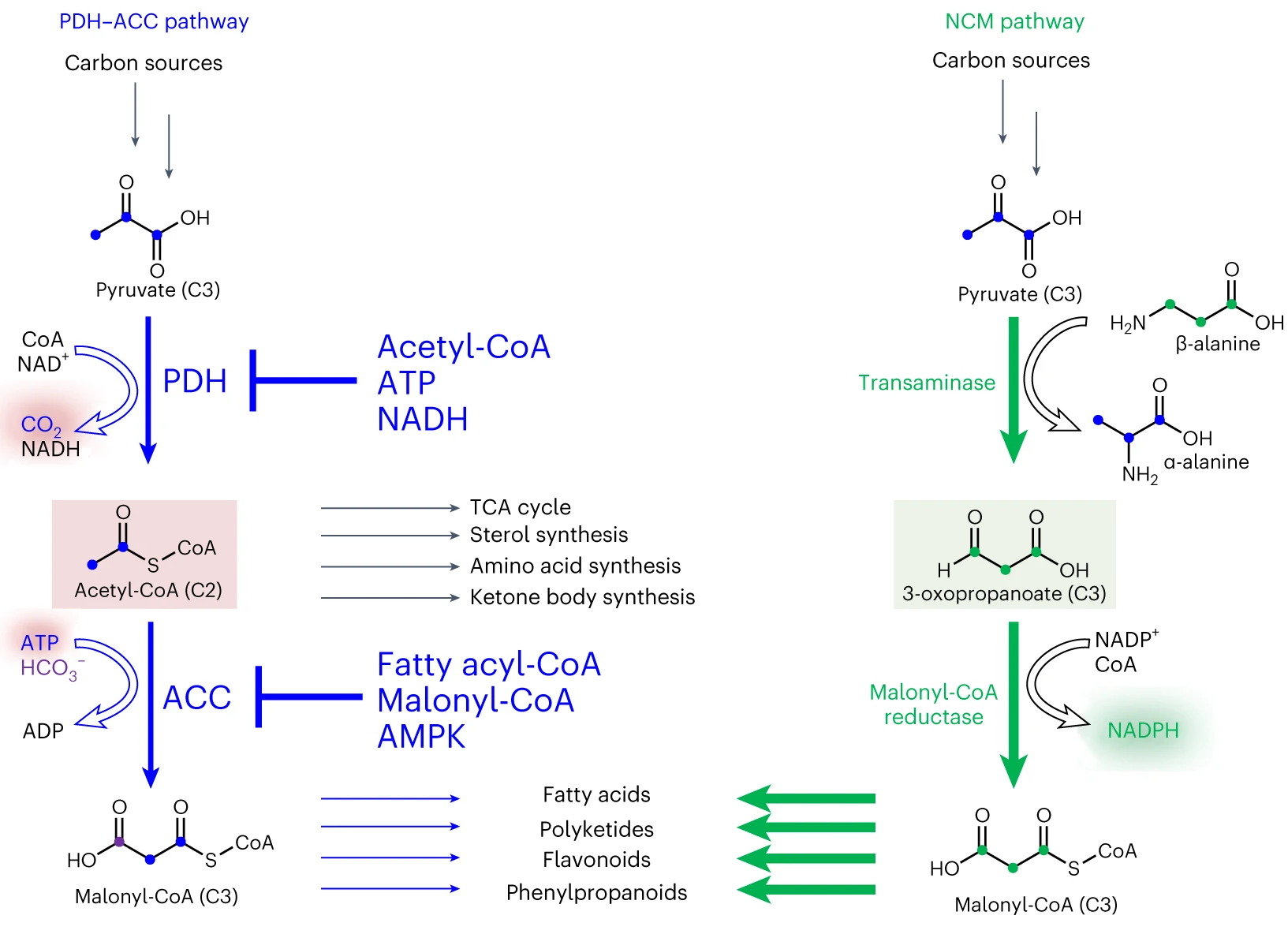 Fig. 1 The natural pathway (PDH-ACC) and NCM pathway for malonyl-CoA biosynthesis.
Fig. 1 The natural pathway (PDH-ACC) and NCM pathway for malonyl-CoA biosynthesis.
To address these limitations, we have developed an artificial pathway termed the non-carboxylative malonyl-CoA (NCM) pathway. The pathway adopts an “acetyl-CoA-independent” mode, thus avoiding carbon loss, ATP consumption and problematic cross-talk with native metabolic networks (Fig. 1). In particular, we utilize 3-oxopropanoate (C3) rather than acetyl-CoA (C2) as an intermediate metabolite between pyruvate (C3) and malonyl-CoA (C3). The feasibility of this design was demonstrated by mining and recruiting β-alanine-pyruvate transaminase and malonyl-CoA reductase. The NCM catalysis displays a obvious fast kinetics, with the rate being 103-fold higher than that of natural pathway, representing the fastest malonyl-CoA formation rate to the best of our knowledge.
Moreover, the NCM catalysis circumvents both multiple tight regulations and extremely complicated architecture associated with natural pathway, with the mass of comprising enzymes reducing by 93%~97%. Furthermore, in contrast to PDH-ACC, the introduction of the NCM pathway does not cause cytotoxicity and instead improves the robustness of cells. This might be due to the production of reducing power NADPH rather than NADH during NCM catalysis. This finding is particularly valuable in the production of natural products, as it enhances cell tolerance to inhibitory chemicals.
We also tested the application of NCM pathway. In various microorganisms, including model microorganism E. coli, non-model microorganisms Streptomyces gilvosporeus and Saccharopolyspora spinosa, the NCM pathway has been successfully used to increase the yield of different MDPs. In particular, introduction of NCM pathway into S. spinosa enabled the spinosad titer further increasing to 4.6 g/L in shake flasks. To the best of our knowledge, this represents the highest spinosad titer reported in any native or engineered microbes under any cultivation conditions.
In summary, the NCM pathway offers a novel and advantageous alternative to traditional approaches for malonyl-CoA biosynthesis. By circumventing the limitations of the PDH-ACC pathway, the NCM pathway demonstrates improved catalytic efficiency, avoids strict feedback control, and saves carbon sources and energy. Thus, it has the great potential to serve as a universal platform for the synthesis of MDPs with high titer, rate, and yield.
Please find our article with a title of "A non-carboxylative route for the efficient synthesis of central metabolite malonyl-CoA and its derived products" which has been publised at Nature Catalysis (https://www.nature.com/articles/s41929-023-01103-2).
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in