Binodal Organometallic Network Achieved by On-Surface Dissymmetric Reaction
Published in Chemistry
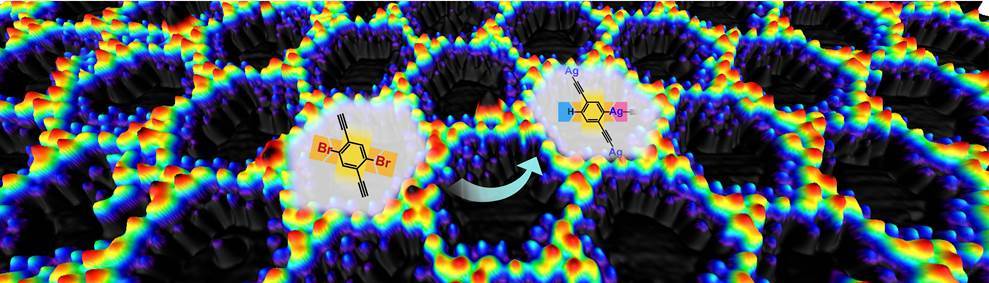
Rapid development of both synthetic chemistry and materials science desperately calls for a comprehensive chemical reaction toolbox which contains both the needed reactions and their fine-tuning methodologies. These methodological strategies are of great significance in augmenting the efficiency and precision of the synthetical chemistry. For example, by careful and efficient control of the reaction process, different reactions may take place on the identical sites within one molecule, then complicated products could possibly come out of very simple precursors. Such a differentiated reaction of equivalent reacting sites within one molecule is termed as “dissymmetric reaction”. However, the realization of this exciting idea via wet chemistry is of great challenge due to the uncontrollability at molecular level in the homogeneous reaction environment. Therefore, we turned to heterogenous reaction environment for a possible solution.
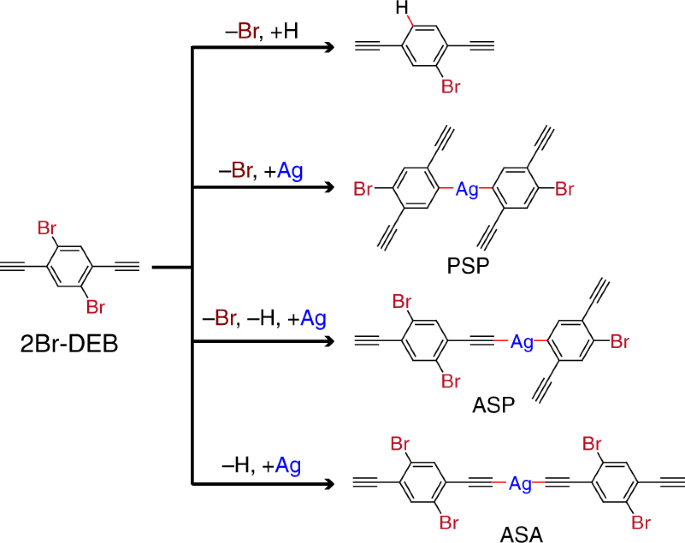
In the past decade, on-surface chemistry has evolved as an efficient approach to synthesizing various organic structures that are not available by conventional synthesis strategy. Inspired by this fact, we decided to try out an on-surface synthesis strategy, aiming at the realization of dissymmetric reaction. We first introduced the metal substrate into the reaction system in the expectation that the heterogenous reaction environment created by the substrate surface would facilitate the differentiation in reactivities of the equivalent groups. Subsequently we designed a bi-functional molecular precursor, 1,4-dibromo-2,5-diethynylbenzene (2Br-DEB), which is obtained by attachment of two terminal alkynyl groups to 1,4-dibromobenzene. The akynyl groups serve as proton donors via their C-H bond cleavage so that both metalation and H-passivation reactions can take place at the equivalent Br-substituted sites in 2Br-DEB.
Based on the carefully designed 2Br-DEB/Ag(111) reaction system, the dissymmetric reaction eventually emerged. Scanning tunneling microscopy imaging at the sub-molecular level reveals that the differentiated functionalization of the two equivalent Br-substituted sites in the precursor takes place in a stepwise manner. The reaction starts from the H-passivation of one Br-substituted site at 300 K in accompany with the intermolecular reaction to form one-dimensional organometallic chains containing alkynyl-silver-alkynyl nodes. Afterwards, the other equivalent Br-substituted site undergoes metalation reactions at 320~450 K, resulting in transformation of the chains into a two-dimensional network which contains two types of organometallic connections, i.e., alkynyl-silver-alkynyl and alkynyl-silver-phenyl nodes.
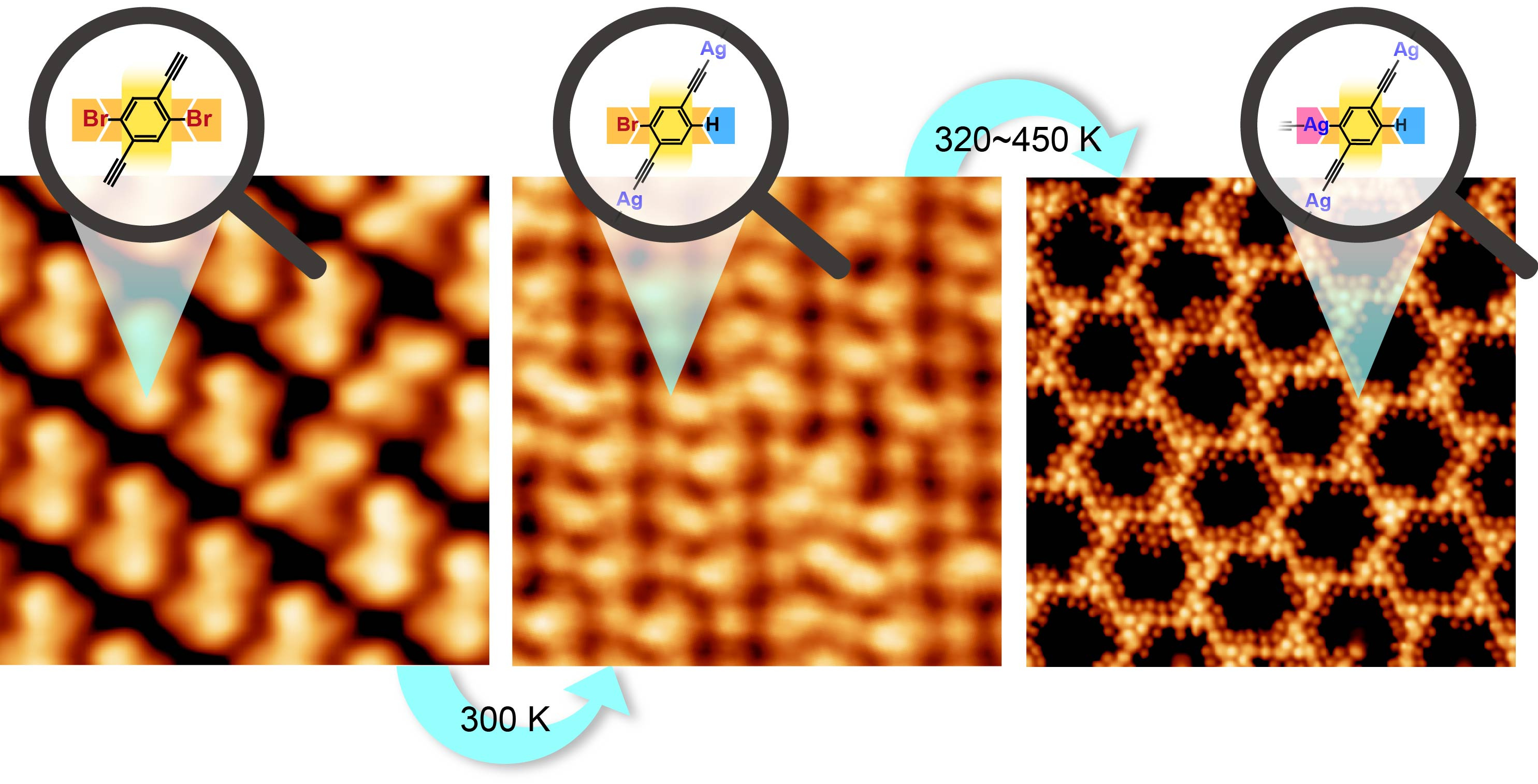
In order to gain an insight into the mechanism of the dissymmetric reaction, we carried out density functional theory calculations. The calculated reaction potential pathways indicate that a much higher activation energy for the detachment of the second Br atom in the molecule than that of the first holds the key to the stepwise activation of the two Br atoms in 2Br-DEB. The computational exploration also discloses an irreversible reaction with H atoms and a reversible reaction with Ag adatoms of the debrominated molecular moieties. These theoretical results can well explain the selective C-H coupling of the debrominated sites at 300 K when abundant H atoms are available due to the concurrent C-H cleavage of the terminal alkynyl groups. Whereas, the molecule-Ag reaction becomes dominant at elevated temperatures due to the exhaustion of the surface H atoms and enhanced surface diffusivities of both the Ag adatoms and molecular moieties.
Our findings not only exemplify the achievement of the dissymmetric reaction by using carefully designed precursors and precisely controlled reaction processes, but also provide a potential solution to the construction of complex nanostructures and macromolecules via dissymmetric reactions. It is our sincere hope that this work would inspire the exploration of other dissymmetric reaction systems that leads to complicated biological or drug molecules and multifunctional materials as well.
To learn more about this work, please check out our article “Stepwise On-Surface Dissymmetric Reaction to Construct Binodal Organometallic Network” published in Nature Communications (https://doi.org/10.1038/s41467-019-10522-4).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in