Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (RIALTO)
Published in Cancer
Like
Liked by nessma shahin
The development of the RIALTO trial (NCT02187354), whose results are now published (Locatelli et al. Blood Cancer Journal (2020) 10:77), was based on 2 key factors:
- Like all blinatumomab protocols, also this study was developed in close collaboration with Cooperative Groups, which, for this population of patients (namely relapsed/refractory childhood B precursor acute lymphoblastic leukemia, BCP-ALL) were IntReALL and COG.
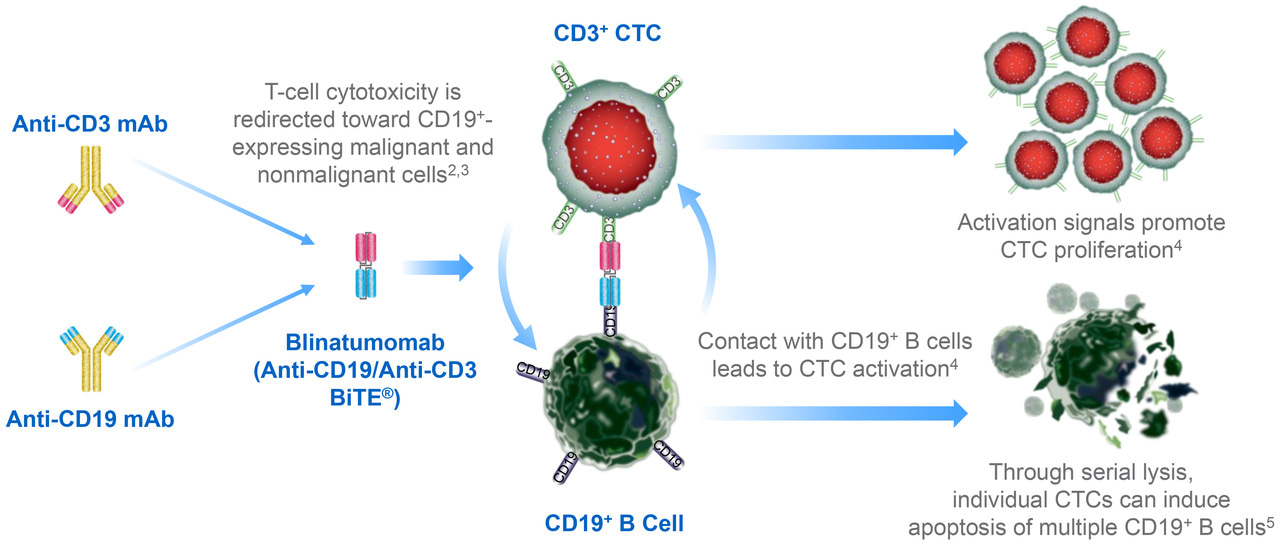
- In children with relapsed/refractory BCP-ALL, like in patients with other ALL indications, 2 trials were conducted. The target populations in the 2 trials were similar, but not identical. Thus, the trials complemented each other, ensuring confirmation of the data. The current trial published in Blood Cancer Journal included the target population of the 1st trial MT103-203 (BLAST; NCT01207388), as well as, in addition, patients with lower tumor burden (i.e. between 5 and 25%) and patients with molecularly resistant disease. This choice allowed to confirm that patients with low leukemia burden have a high chance to benefit from treatment with blinatumomab. Also 1st data of MRD in 2nd relapse could be collected. The efficacy of blinatumomab was confirmed to be independent of the presence of genetic alteration, as shown by the response obtained in patients with t(17;19) and with constitutional Trisomy 21. Noteworthy, patients with t(17;19) have a dismal prognosis and no option of cure by standard treatment. Patients with constitutional Trisomy 21 have a poor tolerance to chemotherapy and may suffer from multiple severe and serious side effects. The agnostic nature of blinatumomab ignoring any genetic alterations and/or pathological changes of the intracellular pathway is illustrated by the cartoon below.
Link to manuscript: https://www.nature.com/articles/s41408-020-00342-x
Follow the Topic
Cancer Biology
Life Sciences > Biological Sciences > Cancer Biology
-
Blood Cancer Journal

This journal seeks to publish articles of the highest quality related to hematologic malignancies and related disorders.
Latest Content
Behind the Paper, Empower Your Research, Bullying in school and in the workplace Hub, ECR Hub, Cancer in understudied populations Hub, Myeloid cell function and dysfunction Hub
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in