Glioblastoma (GBM) is a frightening disease. This incurable tumour invades the highly vascularized brain tissue with a lighting speed and virtually always comes back (recurs) after intensive therapy. Enormous research efforts invested in cracking the GBM quandary revealed that among myriads of heterogeneous cancer cells, one type is particularly critical – the GBM stem cells (GSCs). GSCs are important because they make GBM incurable by giving rise to new tumours after the initial lesion has been decimated by surgery, chemotherapy and radiation. Therefore, understanding the modus operandi of GSCs may offer invaluable therapeutic clues.
It has been known for some time that GSCs come in two flavours. Among them, proneural GSCs resemble normal neural stem cells and form tight clusters (neurosphreres) when cultured in a dish. In contrast, mesenchymal GSCs are elongated, invasive, migratory and highly aggressive in vivo1. It has also been postulated that GSCs mostly reside in the proximity to blood vessel endothelial cells from which they receive complex and somewhat mysterious signals2. The question that has long remained unanswered is whether proneural and mesenchymal GSCs receive and respond to similar or different endothelial messages, and what is their nature. Our report in Nature Communications3 takes on this challenge.
First, we observed that, indeed, GSCs implanted into brains of mice form tumours in which cells proximal to blood vessels seem to look different and acquire spindles. When we co-cultured proneural GSCs with explants from mouse aortae we observed that these cells migrated toward and aligned themselves with endothelial cells of newly formed blood vessels, as if they received some instructive signals. To find out what kind of signals endothelial cells may send to GSCs, the latter were grown in stem cell preserving neurosphere cultures in the presence of endothelial cells or their derived factors contained in purified conditioned media. Remarkably, contact with this material dramatically changed the behaviour of proneural GSC. In the presence of endothelial factors, proneural cells separated from their multicellular spheres (Fig.1), attached to the dish and assumed motile and elongated, mesenchymal appearance. They also lost the expression of proneural GSC markers (SOX2, activated NOTCH) and expressed mesenchymal markers (CD44, VIM).
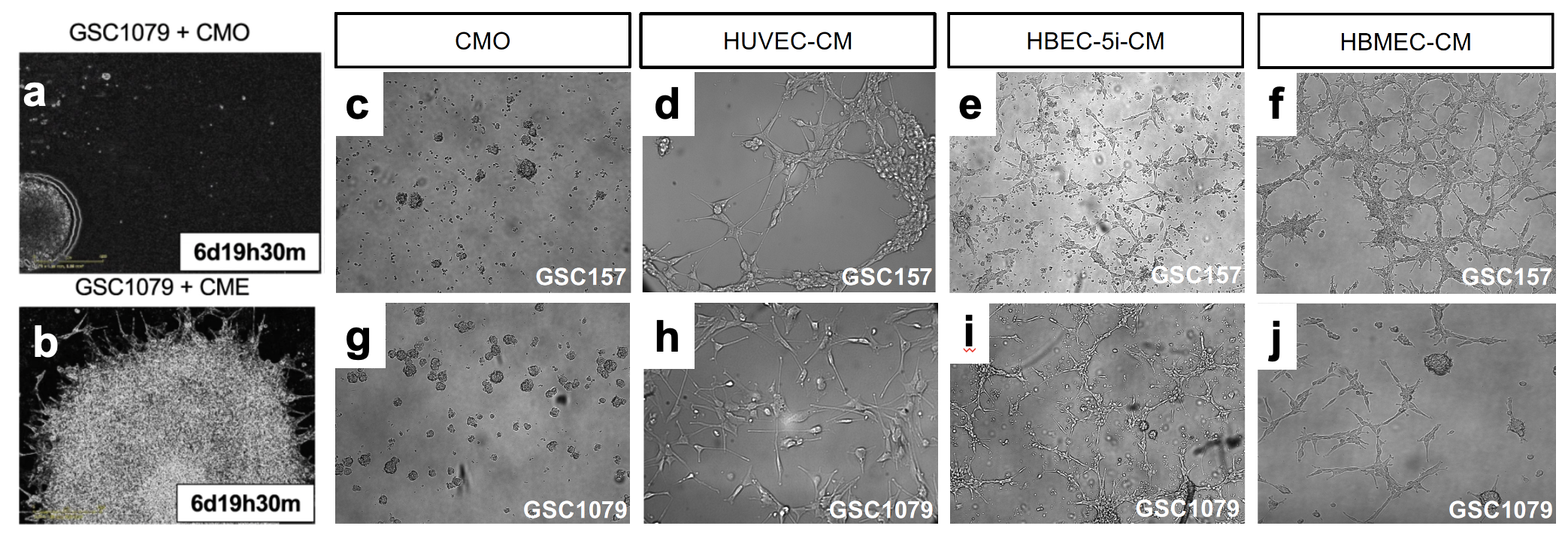
While it was clear that endothelial cells produce something that converts proneural GSCs to mesenchymal-like GSCs, any number of mediators could have produced this effect. What complicates matters in addition to countless growth factors, enzymes and bioactive small molecules is that intercellular communication is often mediated by more complex, multimoleculer entities, such as extracellular vesicles (EVs)4,5. To separate between these possibilities, we fractionated endothelial conditioned media into: EVs and conditioned media deprived of EVs. While endothelial derived EVs (EEVs) had an impact similar to endothelial conditioned media on proneural GSCs, the cells were unperturbed when treated with EV deprived conditioned media (Sup). At a functional level, we observed that EEV treated proneural GSCs develop sensitivity towards chemotherapy relative to controls. This is of significance because proneural GSCs are known to be resistant chemotherapy, relative to mesenchymal GSCs6. Mechanistically, we narrowed down MMPs as the key cargo component carried by EEVs which facilitated the transition of proneural GSCs to a mesenchymal cell fate. We observed that blocking MMP activity pharmacologically, inhibited the decrease of proneural GSC markers (SOX2, activated NOTCH) while also suppressing increase in mesenchymal markers (CD44, VIM and NFkB signaling) in proneural GSCs pretreated with EEVs.
Finally, we identified that a competition exists for EV uptake by proneural GSCs where the cells preferentially took up EEVs relative to their own EVs (OEVs). The proneural to mesenchymal switch was rescued when excessive OEVs were supplied in the presence of EEVs (Fig.2a-c). In mouse experiments, we observed that just 7 days after intracranial injection of proneural GSCs, a bulk cell population at the injection site expressed NICD+VIM- and a cohort of migratory GSCs expressed NICD-VIM+. The bulk cells were enriched in OEVs while the scattered GSCs responded to EEVs. Pre-treatment of proneural GSCs with unfractionated endothelial secretome enabled migration of a greater number of VIM+proneural GSCs in the distinct regions of the brain (Fig.2d). Likewise, a higher number of VIM+ cells were found in vivowhen proneural GSCs were pretreated with EEVs relative to OEVs.
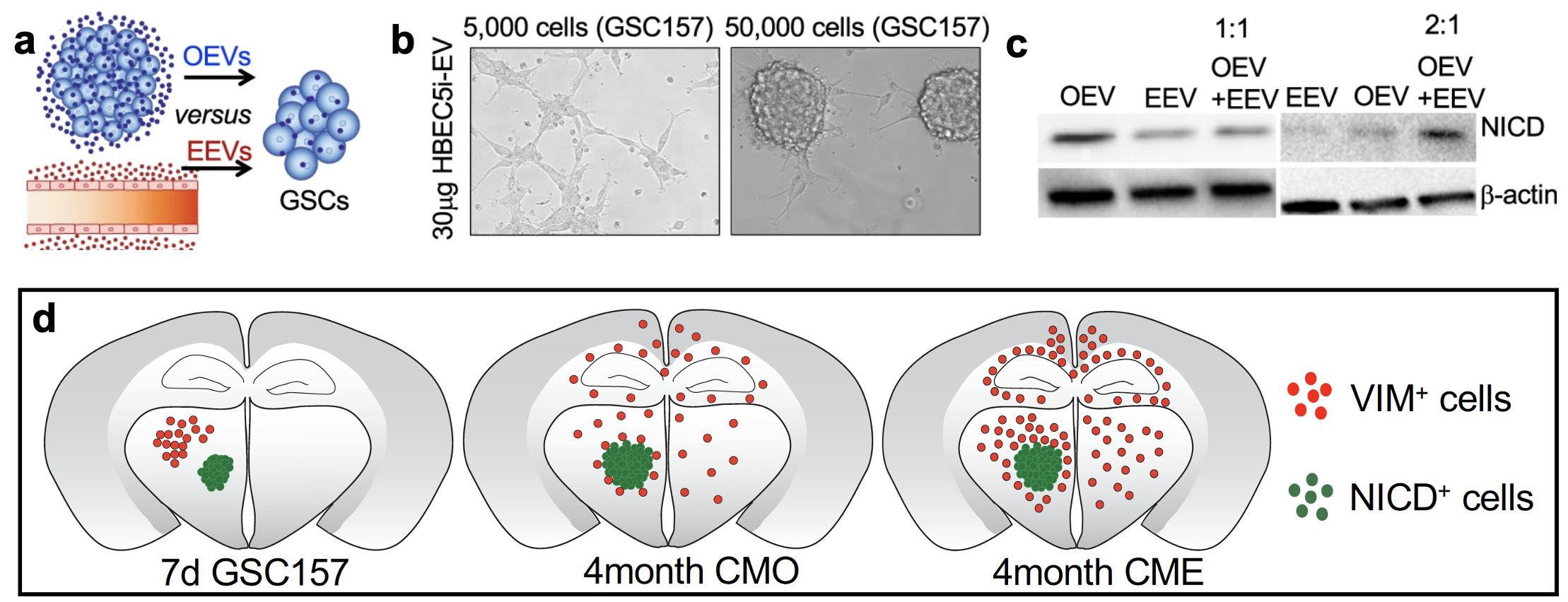
Taken together, we have shown that the vasculature in tumor microenvironment does more than supply nutrients and secrete soluble paracrine signals to facilitate tumor growth in GBM. GSC plasticity is influenced by vesicles secreted from endothelial cells due to the proximity between the two cell types. Our results implicate a need for de novo therapies to modulate endothelial cell vesiculation, EV uptake and/or modulating related signaling responses to better control disease evolution and aggressiveness in a subset of GBM.
References
1 Mao, P. et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A 110, 8644-8649 (2013).
2 Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69-82, doi:10.1016/j.ccr.2006.11.020 (2007).
3 Adnani, L. et al. Angiocrine extracellular vesicles impose mesenchymal reprogramming upon proneural glioma stem cells. Nat Commun 13, 5494, doi:10.1038/s41467-022-33235-7 (2022).
4 van Niel, G., D'Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19, 213-228, doi:10.1038/nrm.2017.125 (2018).
5 Broekman, M. L. et al. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol14, 482-495, doi:10.1038/s41582-018-0025-8 (2018).
6 Garnier, D. et al. Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization. Neuro Oncol 20, 236-248, doi:10.1093/neuonc/nox142 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in