Body weight index indicates the responses of the fecal microbiota, metabolome and proteome to beef/chicken-based diet alterations in Chinese volunteers
Published in Microbiology
Relationships between meat consumption and gut diseases have been debated for decades, and the gut microbiota plays an important role in this interplay. It was speculated that the gut microbiota and relevant indicators of hosts with different body weight indexes (BMIs) might respond differentially to meat-based diet alterations, since lean and obese hosts have different gut microbiota composition. Forty-five young Chinese volunteers were recruited and assigned to high-, middle- and low-BMI groups. All of the volunteers were given a beef-based diet for 2 weeks and subsequently with a chicken-based diet for another 2 weeks. Body weight and blood indexes were measured, and fecal samples were obtained for 16S rRNA sequencing, metabolome and proteome analyses. The fecal metabolites of the low-BMI volunteers showed greater sensitivity to meat-based diet alterations. In contrast, the fecal proteome profiles and blood indexes of the high- and middle-BMI volunteers indicated greater sensitivity to meat-based diet alterations. Replacing the beef-based diet with the chicken-based diet largely changed operational taxonomic units of Bacteroides genus, and thus probably induced downregulation of immunoglobulins in feces. Compared with the beef-based diet, the chicken-based diet decreased inflammation-related blood indexes, especially in high- and middle-BMI volunteers. This work highlighted the role of BMI as an important factor predicting changes in gut homeostasis in response to meat consumption. Compared with the chicken-based diet, the beef-based diet may induce more allergic and inflammation-related responses in high- and middle- BMI Chinese at the current level.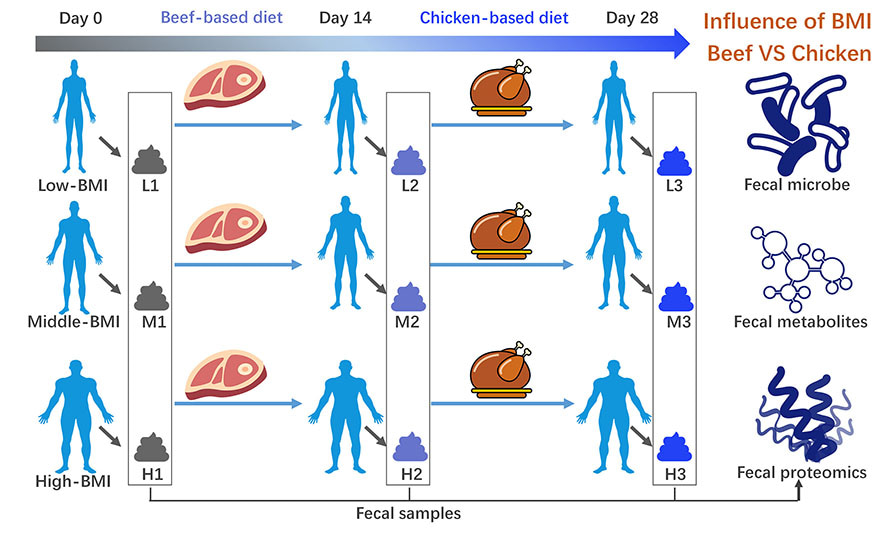
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: Feb 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in