Breaking the Permeability and Selectivity Trade-Off: Ultrathin Polymeric Membranes Achieve Fast and Selective Ion transport

In the quest for better membrane-based separation technologies, scientists have long grappled with a stubborn challenge: creating membranes that are both highly selective and permeable. Traditional polymeric membranes often suffer from a trade-off between the membrane selectivity and permeability. Now, a breakthrough from researchers at the Dalian Institute of Chemical Physics, Chinese Academy of Sciences promises to break the trade-off—with ultrathin polymeric membranes with angstrom-scale channels.
The Problem of Conventional Membranes
Polymer membranes are essential in membrane-based separation technologies, such as flow batteries, fuel cells, water purification and gas separation. Their role in theory allows desired ions or molecules to pass while blocking unwanted ones. In practice, however, most membranes struggle to balance permeability (how quickly ions transport) and selectivity (how well unwanted substances are blocked). Thick membranes prepared by conventional methods (>50 µm) reduce efficiency due to high resistance, while ultrathin membranes often lack mechanical strength or precise pore structures, leading to breakage or leaks.
The Solution: A Two-Step Interfacial Polymer Cross-Linking Strategy
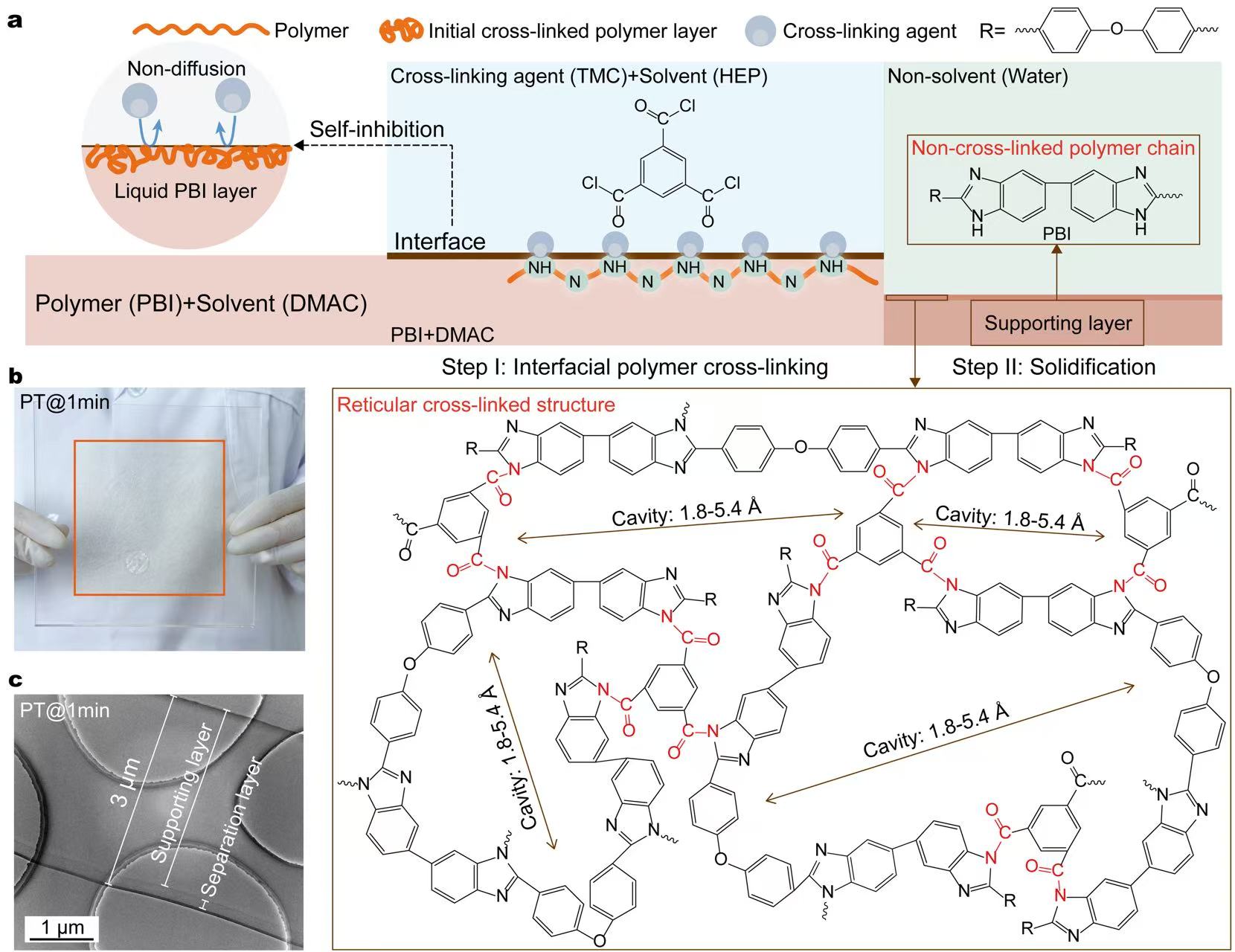
Our team has been committed to the research of membranes in flow batteries for more than 15 years. We aim to reduce the ohmic polarization of flow batteries at high current densities by decreasing the area resistance of the membranes. We developed an interfacial polymer cross-linking method to create membranes down to 3 µm in thick, yet remarkably robust. Here is the preparation process:
- Step I: At the interface of two immiscible solvents, polybenzimidazole (PBI) polymers react with a cross-linking agent, forming a nanoscale separation layer with a quasi-ordered reticular structure. This structure features precisely sized cavities (1.8–5.4 Å), akin to ordered nanoporous crystalline materials.
- Step II: A supporting layer is solidified beneath the separation layer using a non-solvent induced phase separation.
This approach not only reduces thickness but also creates angstrom-scale channels that selectively filter ions based on size and charge.
Performance That Impresses
- Ultrahigh Selectivity: The membranes effectively separate ions as similar in size as charge carriers and redox-active species in a wide pH range, critical for flow batteries. Charged sites within the membrane further enhance selectivity via the Donnan effect, repelling redox-active species with same charges.
- Ultrafast Ion Transport: The area resistance (or the permeability for charge carriers) of our membranes is only ~1/9 of the commercial Nafion 212, which enables rapid ion transport, boosting battery power density.
- Mechanical Robustness: Despite their thinness, the membranes exhibit transverse tensile strength (> 90 MPa) and longitudinal hardness (>300 MPa) superior to commercially available 50-µm-thick Nafion 212 (24.20 MPa and 27 MPa).
Various Aqueous Flow Battery Systems
When tested in vanadium flow batteries, alkaline zinc-iron flow batteries and aqueous organic vanadium-toluidine blue flow batteries, the membranes delivered exceptional results:
- Vanadium Flow Batteries: This battery shows a very high energy efficiency (EE) of 82.38% at an ultrahigh current density of up to 300 mA cm-2.
- Alkaline Zinc-Iron Flow Batteries: This battery delivers an EE of 81.86% at 200 mA cm-2, which is high compared with recently reported AZIFBs.
- Vanadium-Toluidine blue Flow Batteries: This battery attains a high voltage efficiency (VE) of 81.15% and EE of 81.14% at 120 mA cm-2.
Beyond Batteries: A Versatile Future
Our strategy is not limited to PBI polymers or flow batteries. By varying cross-linking agents and polymers, the pore size and structure can be tailored for applications like gas separation, fuel cells, or water desalination. Scalability is another advantage—the method aligns with industrial interfacial polymerization techniques, easing mass production.
Challenges and Next Steps
While the results are groundbreaking, challenges remain. Characterizing the exact cross-linking degree across the entire separation layer and fully understanding ion-transport dynamics require advanced characterizations. Thus, our current goal is to separate the membrane separation layer from the supporting layer so that we can better study the formation mechanism and ion-transport behaviour. The ultimate aim is to further increase the separation performance of the membranes.
If you are curious about our work, you can read our full paper, “Ultrathin membranes prepared through interfacial polymer cross-linking for selective and fast ion transport” published in Nature Chemical Engineering: https://www.nature.com/articles/s44286-025-00238-2
Follow the Topic
-
Nature Chemical Engineering

This is a new monthly online journal dedicated to publishing the most significant original research, commentary and analysis of direct relevance to the diverse community of chemical engineers.
Ask the Editor – Polymers
Got a question for the editor about Functional polymers? Ask it here!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in