Bridging the gap: an all-around platform for evaluating biomechanical thrombogenesis
Published in Bioengineering & Biotechnology, Cell & Molecular Biology, and General & Internal Medicine
Arterial thrombosis describes the formation of pathological blood clots in arteries that can lead to cardiovascular diseases. It remains the leading cause of morbidity and mortality worldwide, despite recent advancements in biomedical and clinical sciences. Notably, there is currently no bioassay available in standard clinical settings to evaluate the risk of arterial thrombosis in the general population. Furthermore, while shear force is well known to facilitate platelet-enriched blood clot formation, this phenomenon is not emphasized in most laboratory and clinical blood test assays.
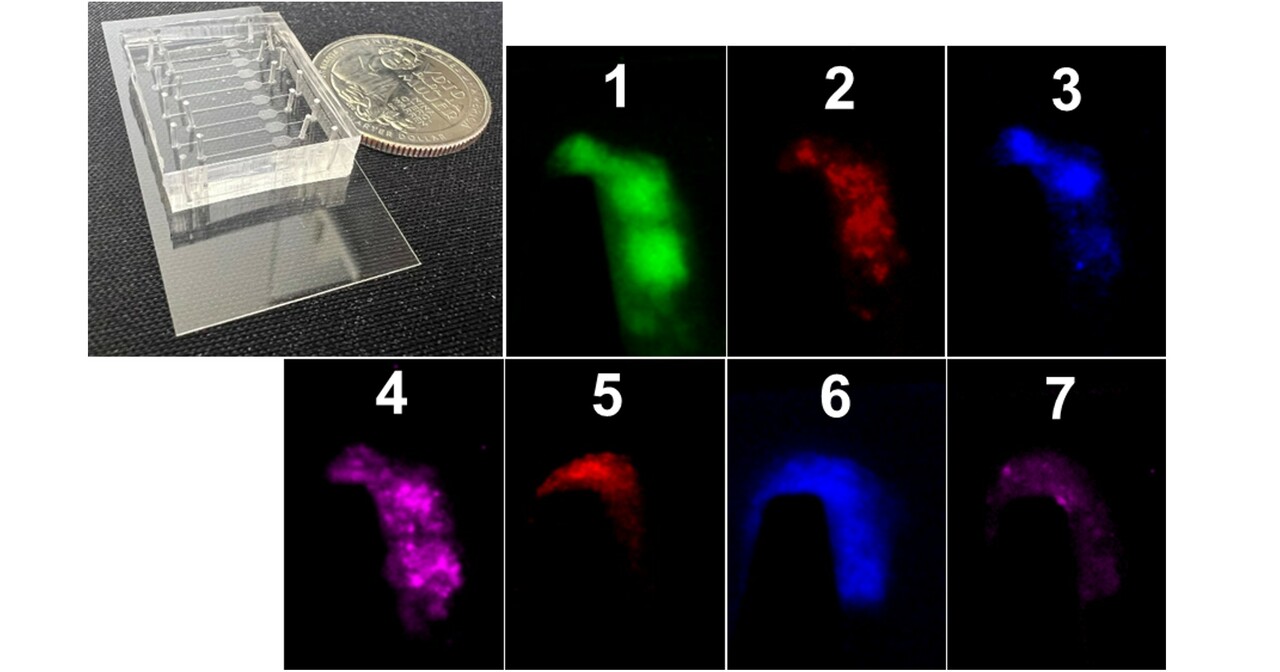
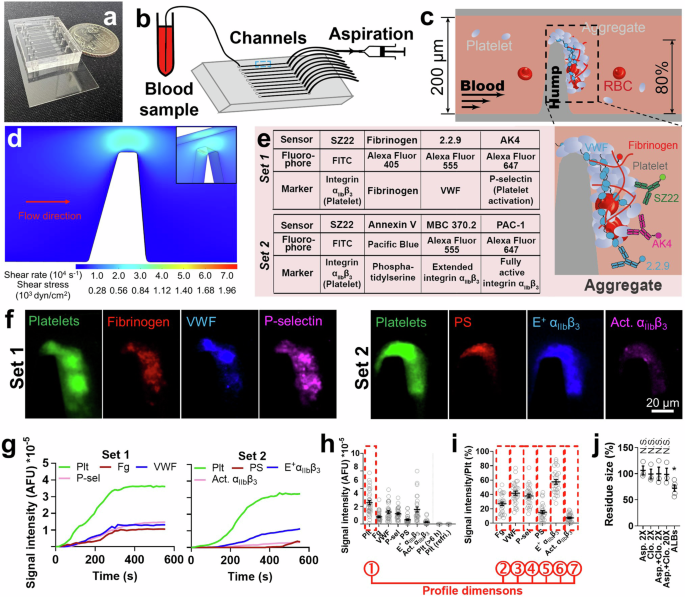
In this work, we developed a thrombus profiling assay that combines multi-color fluorescence imaging with microfluidics, and used it to study how shear-induced platelet aggregation (termed by us as ‘biomechanical platelet aggregation’) contributes to arterial thrombosis. We detected intensified biomechanical thrombogenesis in hypertensive and/or aged individuals, highlighting the potential of our thrombus profiling assay for clinical practice in evaluating thrombotic risks. Moreover, we used a total of 4 complementary assays—laminar flow chamber, biomembrane force probe (BFP), fluorescence BFP and flow cytometry—to decipher part of the molecular mechanisms. Specifically, we identified that hypertension induces the hyperactivity of the GPIbα-integrin αIIbβ3 mechanosensing axis, which directly facilitates biomechanical platelet aggregation and contributes to the higher risks of cardiovascular diseases in those patients.
“Many people are claiming that platelet mechanobiology contributes to arterial thrombosis, and it is indeed an easy guess,” says senior author Yunfeng Chen, PhD, assistant professor of biochemistry and molecular biology at The University of Texas Medical Branch. “However, while abnormal platelet aggregation contributing to higher risks of arterial thrombosis is well acknowledged, whether it involves abnormal biomechanical platelet aggregation was never investigated. On the other hand, although high shear flow is known to trigger platelet aggregation for decades, all previous works only used healthy human’s blood for testing. To our knowledge, our work is the first that provides a direct linkage between high thrombotic risk factors (e.g., hypertension, diabetes, cancer, aging, etc.) and intensified biomechanical platelet aggregation.”
Furthermore, we explored the application of a thrombus "barcode system" for evaluating the pathological phenotype of subjects as well as the effect of antithrombotic drugs. A patient's thrombus barcode may help clinicians determine a matching drug or combination of drugs that allows higher efficacy with fewer side effects. We suspect that the optimal drug/regimen will vary from patient to patient, necessitating the development of a library of antithrombotic agents to meet diverse patient needs. The barcode system also offers important applications in drug screening and mechanism discovery, because it enables us to use the effect barcode of an uncharacterized drug to reason backward its therapeutic target and mechanism of action.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in