Carbonation and modeling study for process liquor in batch mode using fue gas in the mining and mineral processing industry
Published in Chemistry, Earth & Environment, and Mechanical Engineering
The mining and mineral processing industry generates significant huge amounts of flue gas containing CO2 which is a major cause of the greenhouse effect leading to global warming. This work presents the experimental feasibility study of the carbonation where process liquor is contacted with boiler flue gas as a source of carbon dioxide (CO2). It is in the context of the low-grade mineral ore benefaction process. The carbonation process results in the production of stable carbonate or bicarbonate compounds that can be used as valuable by-products or raw materials in other industrial processes. Trials were conducted for the effective carbonation of process liquor generated in the mineral processing industry, which contains caustic lye. This work presents insight into experiments conducted for the carbonation of process liquor and further reaction modeling. The results were found to encourage pursuing large scale columns for carbonation or capture of CO2 into alkali liquor in process industries.
In the present climatic changes world over, there is a significant concern about global owing to the use of fossil fuels. Carbon dioxide generated from various industrial equipment like boilers has a significant global warming potential. A remedy for this is to capture or convert CO2 into other benign forms. In this work, the intention is to capture the CO2 present in flue gases generated from the smoke stack by contacting the flue gas with an alkali solution like NaOH in water which is also a process stream. Hence, two goals will be achieved with a single process; one is to capture and the other is to convert CO2 into sodium carbonate which can be recycled as a process reagent in ore benefaction. This work and its findings can be extended to other industries also. Since CO2 is a significant contributor to global warming, it will help if the CO2 generated in industries is captured and converted to other chemical forms thereby reducing its effect on the atmosphere. Control over carbon dioxide (CO2) emissions from industries is of paramount importance for several reasons.
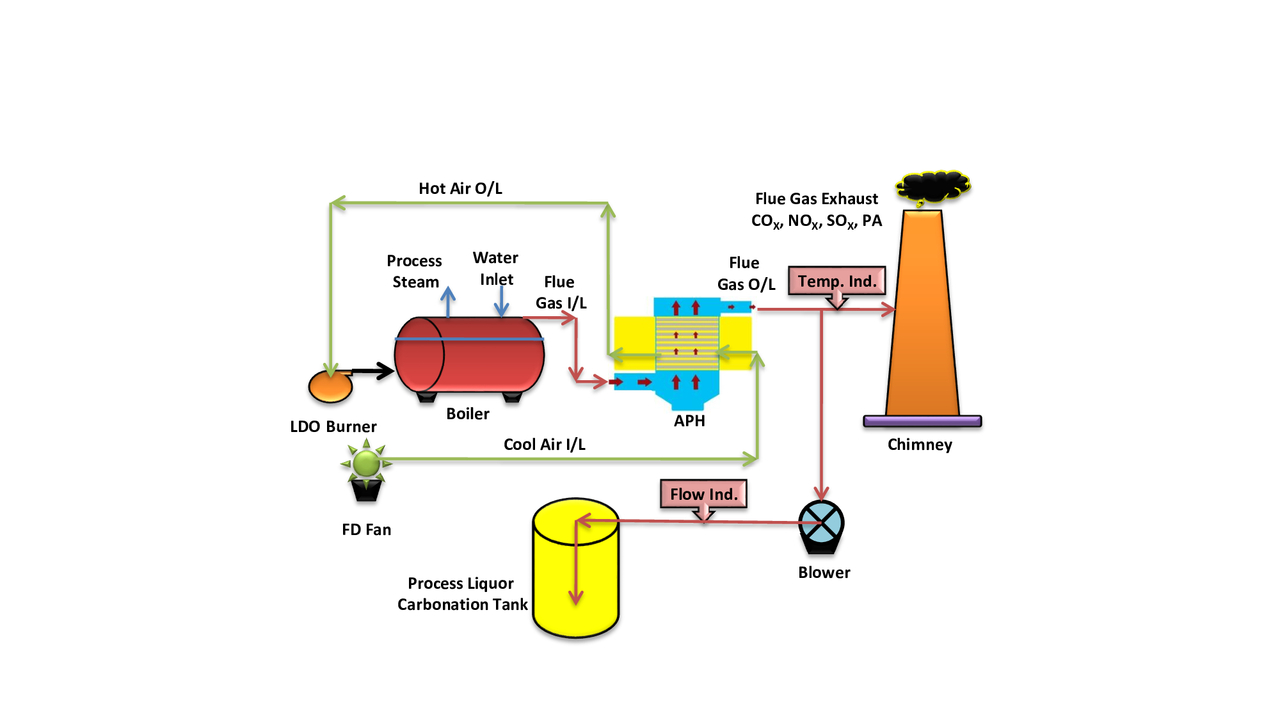
Various leachants used in low-grade ore mineral processing plants are, namely sodium carbonate as an alkaline leachant for phosphatic siliceous dolostone rock and sulfuric acid for uraninite and pitch blend ore (Rao 2019; MoEF&CC 2017). A combination of oil-fired boiler, air preheater (APH), and stack has been provided in technical paper. The experimental setup was established at the mineral processing unit for trials regarding the carbonation of process liquor in batch method. Table is provided for the main parameters of carbonation trials. APH is used for increasing the temperature of boiler inlet air. The average temperature of the flue gas outlet is 203 °C. Heat exchange takes place in APH because from one side cold air is used to pass and hot flue gas received from the boiler stack is used to pass from another side of APH. In this way, heat transfer takes place inside APH. We took one outlet from the flue gas passage of APH to the chimney. One blower was installed for blowing flue gas inside process liquor in batch method. Samples were collected and analyzed within regular time intervals. A polymer cane of 5 L was filled with processed liquor. Flue gas passed inside the cane. Samples were collected at regular time intervals of 30 min, and analysis of Na2CO3, NaHCO3, and NaOH was done.
Based on the analysis data obtained, necessary calculations were performed to determine the concentration or purity of sodium carbonate, sodium bicarbonate, and sodium hydroxide in the sample. Results were reported in the appropriate units and format.
Standard laboratory safety protocols were followed while performing the analysis, including the use of personal protective equipment (PPE) and proper handling of chemicals. It is important to note that this is a general outline, and the specific procedures and techniques may vary depending on the laboratory's resources, equipment, and the purpose of the analysis.
Carbonation of chemical processing liquor in the mineral processing industry typically involves the addition of CO2 gas to the liquor to promote the precipitation of impurities. This process is commonly used to remove calcium and magnesium ions from the liquor, as they can interfere with downstream processes or affect the quality of the final product. In terms of using a light diesel oil-fired boiler for carbonation, it is important to note that the primary purpose of the boiler is to generate steam for various heating and process requirements in the mineral processing plant. The carbonation process itself involves the introduction of CO2 gas into the processing liquor, and the boiler is not directly involved in this step. However, the boiler may indirectly support the carbonation process by providing the necessary steam for other aspects of the operation. For example, steam can be used to heat the chemical processing liquor before or during the carbonation process, ensuring optimal conditions for precipitation reactions to occur.
By the performance of experiments involving the purging of entire flue gas into the alkali process liquor, it was found that the CO2 is effectively captured by reactive absorption. One observation was that it was an exothermic reaction. Additionally, it is worth mentioning that carbonation of process liquor using light diesel oil-fired boiler flue gas is a feasible technique employed by industries to reduce CO2 emissions. The reaction kinetic parameters are obtained for multi-step alkali domain reactions leading to the capture of CO2 into reacted forms of sodium carbonate. The scope of suitable large-scale continuous equipment designing, i.e., absorber is proven to be feasible since in this study the relevant kinetic parameters of carbonation are obtained. Trials on pitchblende and uraninite ore are unique in this study which can further open the door toward carbon-free plants and on great step toward reducing global warming. Trials for carbonation and modeling studies for the usage of process liquor in continuous mode using flue gas in the mining and mineral processing industry have a future scope of research.







Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in