Stress-induced brain extracellular vesicles as molecular messengers of resilience
Published in Biomedical Research
 When this project began, our initial interest lay in whether microRNAs (miRNAs) contained in brain-derived extracellular vesicles (BDEVs) might change according to an organism’s stress state. However, as we progressed, a deeper question emerged:
When this project began, our initial interest lay in whether microRNAs (miRNAs) contained in brain-derived extracellular vesicles (BDEVs) might change according to an organism’s stress state. However, as we progressed, a deeper question emerged:
Could the brain itself release molecular messages that help protect it from stress?
This seemingly simple hypothesis reshaped the trajectory of our research.
A turning point: An unexpected discovery
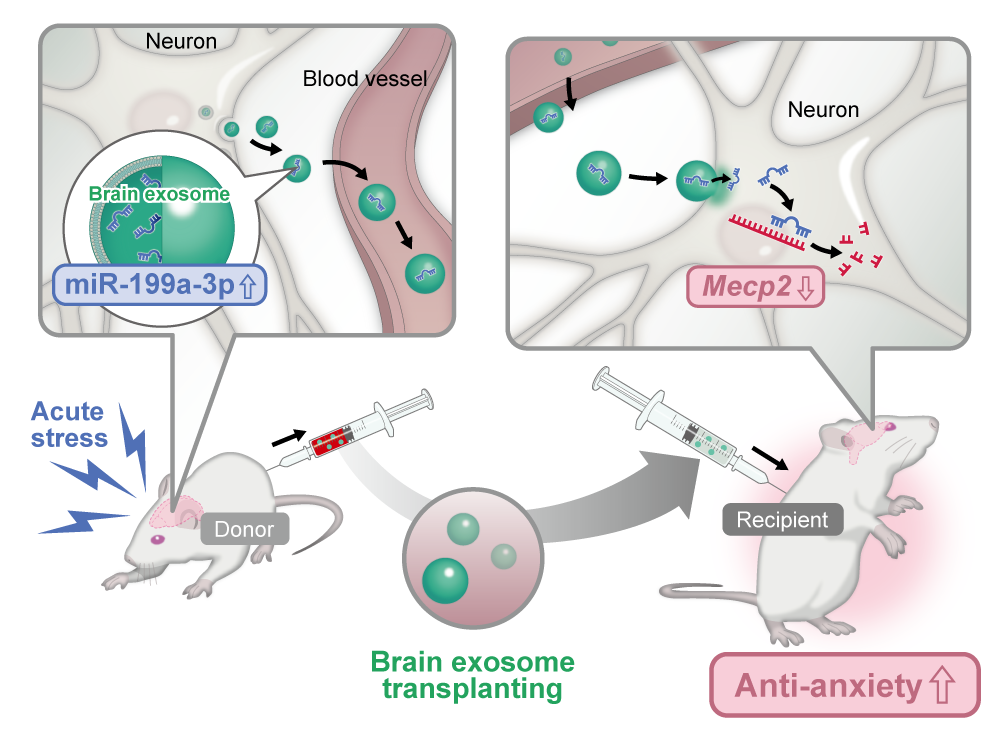
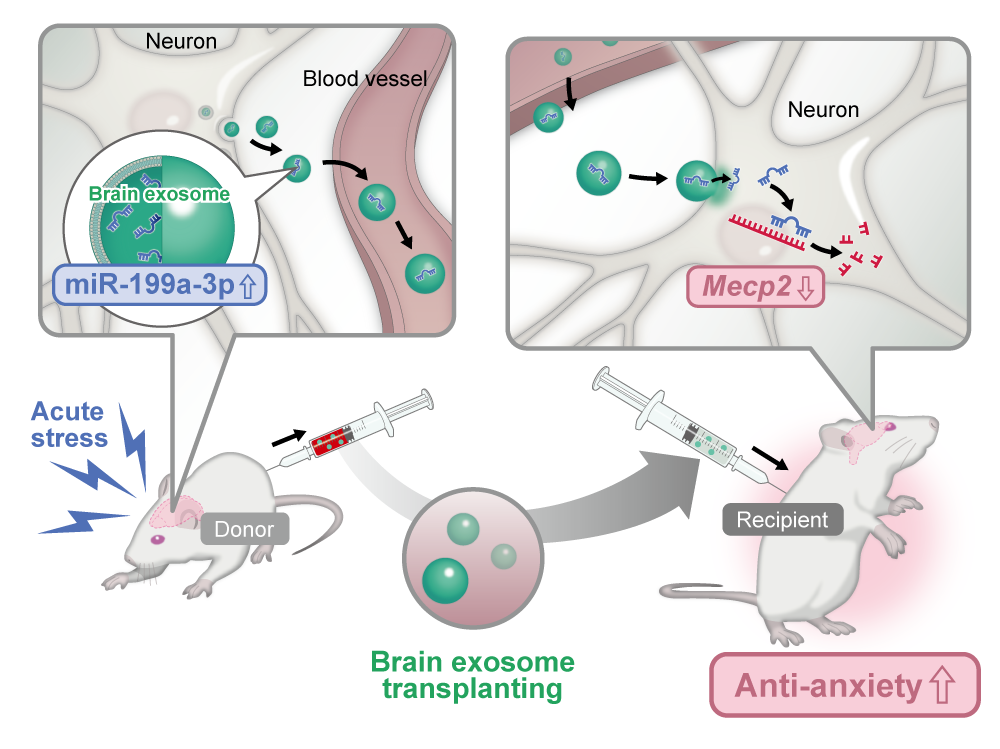
To address this question, we collected BDEVs from mice exposed to acute stress and administered them to naïve animals. Surprisingly, instead of exacerbating anxiety-like behavior, the vesicles alleviated it. The idea that stress might induce the release of “protective EVs” ran counter to conventional thinking and compelled us to reconsider our assumptions.
We had previously identified several miRNAs—most notably miR-199a-3p, miR-99b-3p, and miR-140-5p—that were consistently elevated in BDEVs following acute stress. Yet their physiological significance remained unclear. Integrating these observations with the behavioral findings led us to a compelling hypothesis: these miRNAs might act as mediators of resilience by restoring neuronal and behavioral homeostasis.
Subsequent experiments confirmed that miR-199a-3p played a pivotal role. It acted through the regulation of Mecp2, a gene crucial for neuroplasticity and emotional regulation. This finding not only identified the molecular core of the phenomenon but also offered a framework to understand how stress adaptation may be encoded and transmitted.
Behind the scenes: Building an interdisciplinary bridge
The path toward this conclusion was anything but linear. The limited yield of BDEVs required refinements in isolation techniques and experimental design. Testing the functional contribution of each miRNA demanded iterative assessments and rigorous behavioral evaluation. Rather than being a single breakthrough, the project evolved through countless small decisions—each one sharpening our understanding of what resilience might mean at the molecular level.
Perhaps the most profound challenge was infrastructural. Our laboratory initially lacked the tools and expertise required for EV research. Establishing the necessary platforms—vesicle isolation, molecular profiling, miRNA manipulation—felt like constructing a new language to describe a phenomenon no one had fully articulated. Collaboration became essential. Discussions with experts in EV biology and behavior provided conceptual clarity and guided us toward a coherent mechanistic narrative. Each specialty contributed a missing piece, illustrating how scientific progress often emerges from intellectual cross-pollination rather than isolated advances.
Toward a new view of stress
The implications of our findings extend beyond a single molecular pathway. They suggest that stress is not merely a trigger for neuronal damage but also a signal for adaptive communication. BDEVs may act as messengers of resilience, transmitting molecular instructions that modify neural circuits and behavioral outcomes.
This perspective reframes stress from a purely deleterious force into a dynamic process wherein vulnerability and protection coexist. Understanding how the brain encodes and distributes resilience factors may open new avenues for identifying biomarkers or designing interventions for stress-related disorders.
Looking ahead
Our work leads to a broader question: how far do these vesicular messages travel, and how deeply do they shape the organism’s behavioral landscape? Answering this will require tracing their cellular targets and determining whether similar mechanisms operate in humans.
Scientific discovery often begins with an unexpected observation, but it endures through perseverance, iteration, and dialogue. This project reminded us that biology does not always reveal answers directly; sometimes, it offers hints disguised as anomalies. In following those clues, we learned that the brain may possess an intrinsic strategy for self-protection—one that operates silently, at a scale invisible to the naked eye.
Sometimes, resilience is not taught—it is whispered from cell to cell.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Related Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in