Cardiac lymphatics in Heart Failure
Published in Research Data, Biomedical Research, and Education
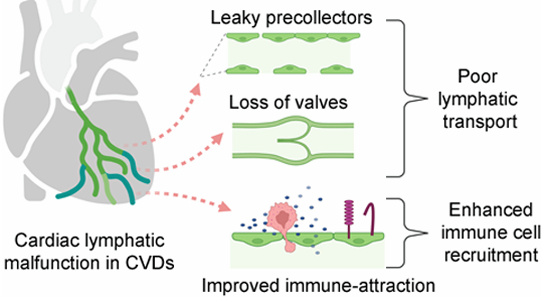
Emerging work in lymphatic biology has highlighted the critical role of immune cells and inflammatory mediators in shaping both lymphangiogenesis and lymphatic vessel function. Conversely, myocardial inflammation is a major therapeutic target in cardiovascular diseases, including Heart Failure. Building on this, we hypothesized that the inflammatory milieu present in the failing heart could reprogram cardiac lymphatics, diminishing their drainage capacity and thereby contribute to chronic myocardial edema, persistent inflammation, and ultimately fibrosis driving Heart Failure development.
To explore how cardiac lymphatic molecular profiles may change during Heart Failure, we launched a collaboration with a team of cardiac single-cell RNA-seq experts led by Prof. Alma Zernecke in Würzburg. Through our jointly funded project, CITE-LYMPH, we established a workflow for single-cell transcriptomic profiling of the exceptionally rare lymphatic endothelial cells (LECs) in the heart. The project also involved close collaboration with local FACS specialists to isolate these cells, as well as next-generation sequencing and bioinformatics teams at Rouen University Normandy to identify transcriptional differences between healthy and failing LECs.
In our study, just published online (Molecular determinants of cardiac lymphatic dysfunction in a chronic pressure-overload model | EMBO Molecular Medicine), we show that myocardial inflammation, particularly signaling through interleukin-1β, profoundly shifts cardiac LEC gene expression. These changes include reduced levels of several key vascular barrier genes (such as Cldn5, Tjp1, and Plvap), suggesting alteration of lymphatic permeability. Because lymphatic vessels are essential for clearing interstitial fluid and solutes to the lymph nodes, modifications of lymphatic barrier function in the heart likely contributes to the chronic myocardial edema observed in our experimental mouse model of Heart Failure.
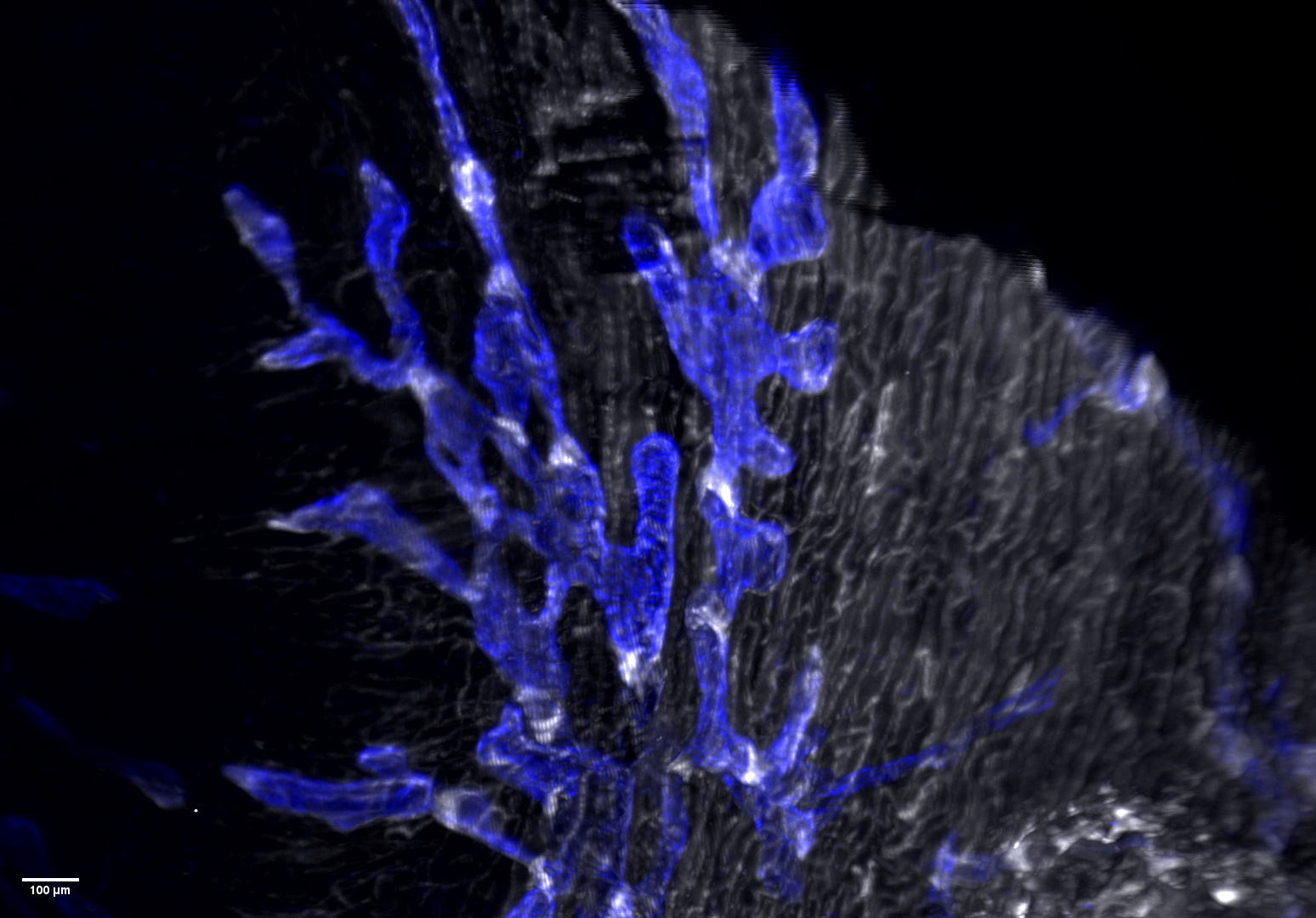
Further analysis revealed striking alterations in LEC subpopulations. One cluster, corresponding to lymphatic valve cells (see beneath), was markedly diminished in diseased hearts. Using lightsheet 3D imaging, we confirmed a loss of valves within cardiac lymphatic capillaries during myocardial inflammation.
We anticipate that these data, which represent the first successful characterization of cardiac LEC subtypes, will be valuable not only to lymphatic biologists studying organ-specific vessel specialization but also to cardiovascular researchers seeking new therapeutic strategies to limit myocardial inflammation and Heart Failure. Our findings raise several important questions: How does inflammation trigger valve loss in lymphatics? Can lymphatic barrier integrity be restored therapeutically? Most critically, does lymphatic valve rarefaction occur in the heart or other tissues in Heart Failure patients? Answers to these questions could reveal new avenues for combating hallmark features of Heart Failure, including congestion in the lungs, extremities, and visceral organs.
Follow the Topic
-
EMBO Molecular Medicine

EMBO Molecular Medicine (EMM) publishes breakthrough research in translational and biomedical sciences in the field of experimental medicine.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in