Catalysis‑Induced Highly‑Stable Interface on Porous Silicon for High‑Rate Lithium‑Ion Batteries
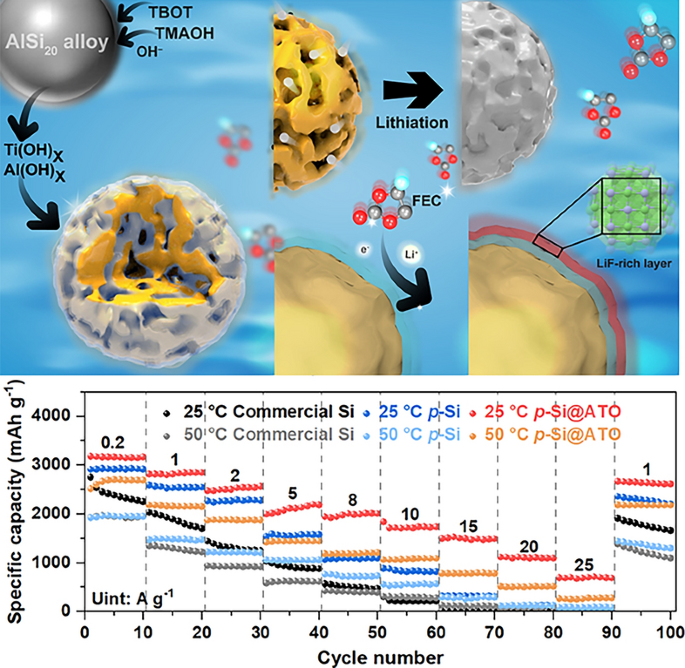
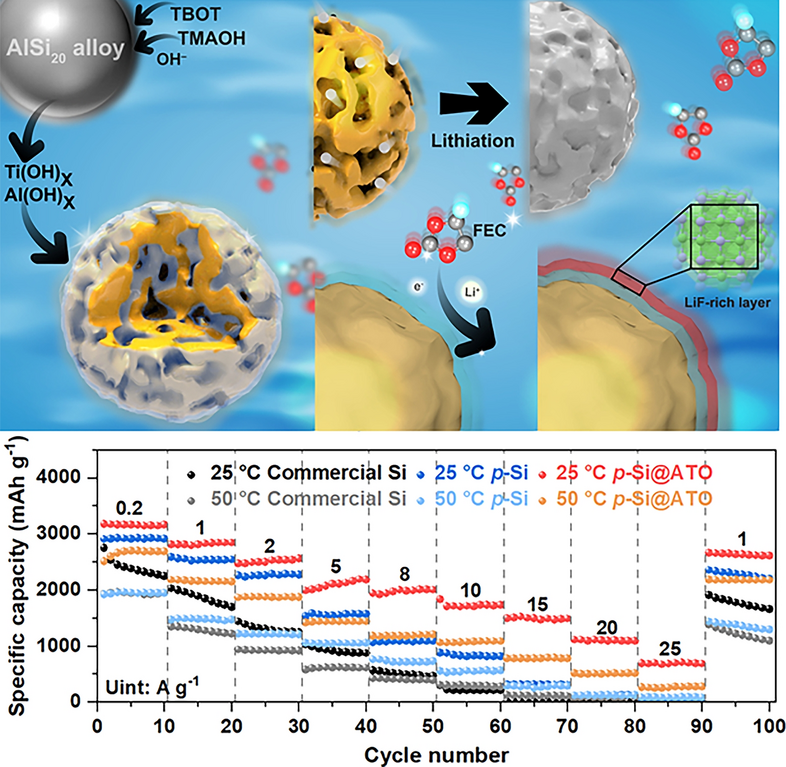
Silicon anodes promise 3,579 mAh g-1—ten times the capacity of graphite—but swell 300 % on each charge, ripping the solid-electrolyte interphase (SEI) and killing batteries within a few hundred cycles. Now, researchers from Shanghai University, Fudan University and partners, led by Dr. Yingying Lv and Prof. Yongyao Xia, have added a 3-nm “catalytic skin” that forces the electrolyte itself to build a rock-solid, LiF-rich SEI exactly where it is needed. The result: a porous-Si particle that survives 1,000 cycles at 20 A g-1 and still delivers 692 mAh g-1 at 25 A g-1—rates that fry conventional Si powders.
Why the Catalytic Interface Matters
• Molecular Concentration → In-situ Conversion: Defect-rich Al–Ti oxide Lewis-acid sites selectively adsorb fluoroethylene-carbonate (FEC) inside 4.9 nm mesopores, pre-concentrating it before catalytic cleavage into LiF.
• Self-Healing SEI: LiF content rises from 90.6 → 93.1 at% during cycling, locking out fresh electrolyte attack and suppressing parasitic reactions.
• High-Temperature Resilience: At 50 °C the same particle retains 80 % capacity after 500 cycles—double the life of commercial Si—while Coulombic efficiency stays > 98.9 %.
Innovative Design & Features
• One-Pot Synthesis: AlSi20 alloy microspheres are mildly etched in alkaline TMAOH while TBOT hydrolyses; Al dissolves, Si porifies, and an amorphous Al–Ti–O layer co-grows in situ—no extra vacuum steps.
• 3–5 nm Oxide Skin: EPR and XAS confirm Ti3+/oxygen-vacancy pairs that lower the d-band centre, accelerating C–F bond scission and LiF precipitation.
• Bimodal Pore Architecture: 4.9 nm mesopores buffer volume expansion; 50–80 nm macropores shorten Li+ diffusion paths, raising the effective diffusion coefficient by 100× versus dense Si.
Applications & Future Outlook
• Extreme Fast-Charge: Half-cells deliver 1,730 mAh g-1 at 10 A g-1 and 692 mAh g-1 at 25 A g-1—enabling 5-min charge without pre-lithiation.
• Practical Pouch Cells: A 3.2 mAh cm-2 p-Si@ATO || LiFePO4 pouch retains 77.4 % capacity over 50 cycles with 97.5 % average CE, validating scale-up.
• Beyond Silicon: The “adsorb-then-catalyse” concept is substrate-agnostic; the same surface chemistry is being rolled out to micro-Sn and micro-Sb anodes for sodium-ion packs.
Challenges & Opportunities: The team is now optimizing Ti/Al ratio for 1,500 °C cycle abuse tests and pilot-line slot-die coating to meet automotive 10-min fast-charge targets.
This work rewrites the silicon-anode playbook—instead of fighting SEI fracture, let the electrode build its own armour. Stay tuned for more gigafactory-ready breakthroughs from Dr. Yingying Lv and Prof. Yongyao Xia!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in