α-Amino acids are essential building blocks of life. It is therefore not surprising that there is a high demand for all kinds of unnatural and non-proteinogenic α-amino acids to modulate the chemical, physical, and pharmaceutical properties of peptides, proteins and other bioactive molecules. Beyond biological applications, chiral α-amino acids are also used as chiral building blocks for chiral catalysts, chiral auxiliaries and diverse (macro)molecules. Synthetic chemists have therefore developed many powerful methods to satisfy the increasing demand for optically active α-amino acids. However, an efficient and economical catalytic enantioselective method is still thought after.
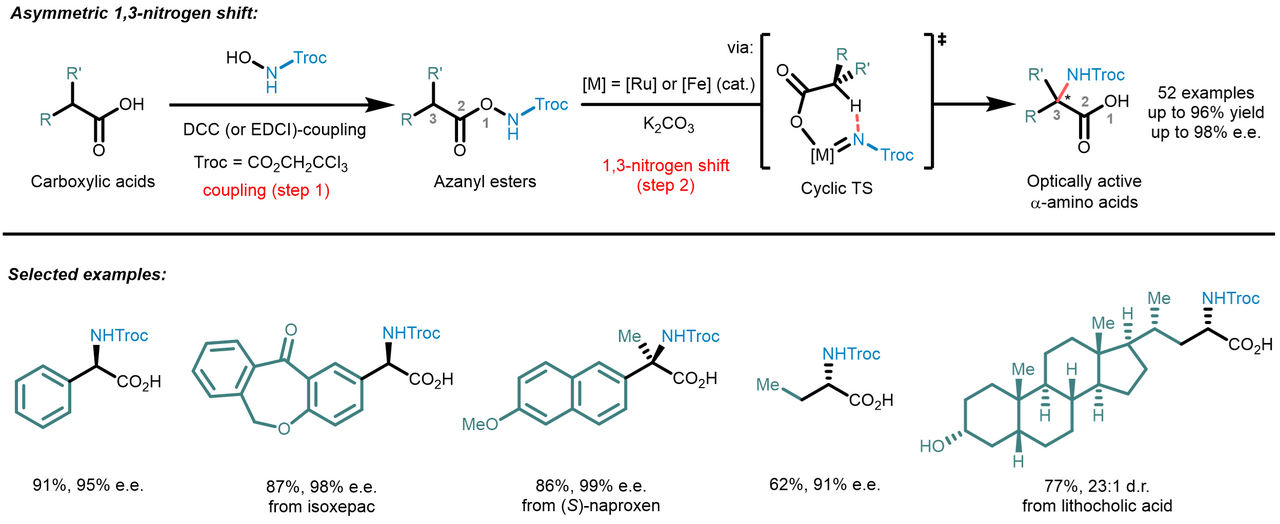
Our new 2-step method is surprisingly simple and straightforward: Abundant carboxylic acids are ligated to a nitrene precursor followed by a stereocontrolled 1,3-nitrogen shift from the carboxylic acid oxygen to the α-carbon. Mechanistically, after ligation to an azanyl ester, the transition metal catalyst (ruthenium or iron) inserts into the O-N bond to form a transition metal nitrene which undergoes a stereocontrolled intramolecular C(sp3)-H insertion into the coordinated carboxylate fragment. The resulting Troc-protected amino acids obtained through this protocol can be used directly for further applications with the Troc-group being selectively removable under mild conditions via a reductive Grob fragmentation.
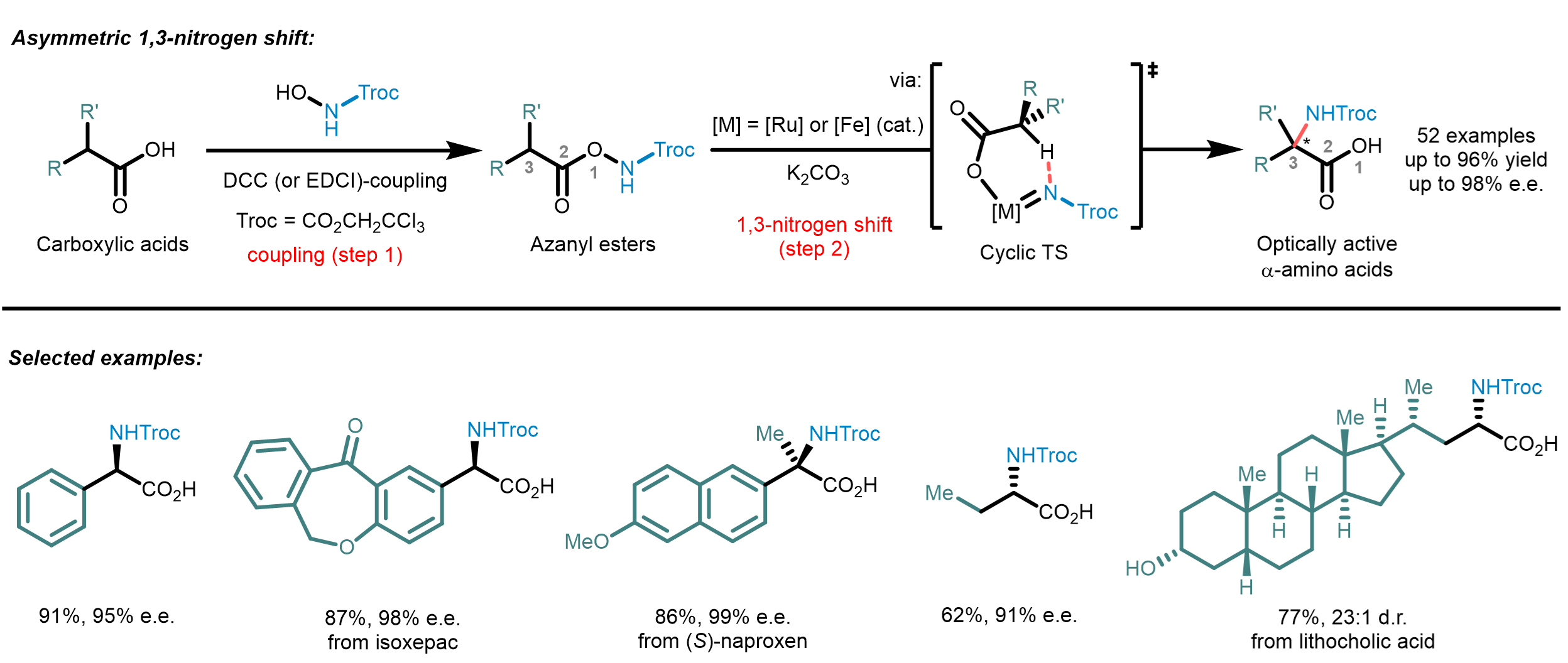
Inspiration for this work came from recent progress in nitrene chemistry. Transition metal catalyzed nitrene C(sp3)-H insertion is a powerful tool for the stereocontrolled introduction of C-N bonds into organic molecules. However, to ensure high regio- and stereocontrol, such C(sp3)-H aminations are typically performed as intramolecular ring-closing reactions, thus not allowing to synthesize acyclic compounds such as amino acids. Our new method combines the advantages of an intramolecular nitrene insertion with the ability to access acyclic amine-containing molecules by using an unprecedented 1,3-migratory nitrene C(sp3)-H insertion mechanism.
The developed catalytic enantioselective α-amino acid synthesis has several attractive features: It employs abundant and easily accessible carboxylic acid feed stock molecules, provides chiral α-amino acids with a protection group that can be removed under mild reaction conditions, the nitrogen shift provides excellent stereocontrol, and the nitrogen shift can even be catalyzed with a nontoxic and robust iron catalyst. This straightforward method displays a very broad scope, providing rapid access to optically active α-amino acids with aryl, allyl, propargyl, and alkyl side chains, and also permits stereocontrolled late-stage amination of carboxylic acid-containing drugs and natural products. We believe that this work will expedite the synthesis of unnatural α-amino acids and boosting the development of related fields.
For more details, see our paper: "Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids". Link: https://www.nature.com/articles/s41557-022-00895-3.
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in