CD44 is a regulator of cell plasticity
Published in Cancer

Back in 2020 we reported CD44 to be a crucial protein in cancer cell plasticity by mediating iron endocytosis, which we wrote about in the Chemistry Community before. CD44 is a cell surface protein found in many cell types besides the cancer stem cell niche (sometimes also referred to as the persister cancer cell niche). Importantly, it is used in many settings as a "marker" for activated cells, including immune cells such as dendritic cells, macrophages and T cells. Indeed, it has been known since 1990 that CD44 is the principle receptor for hyaluronic acid in cells1 and takes up the glycan via endocytosis2.
What does CD44 do?
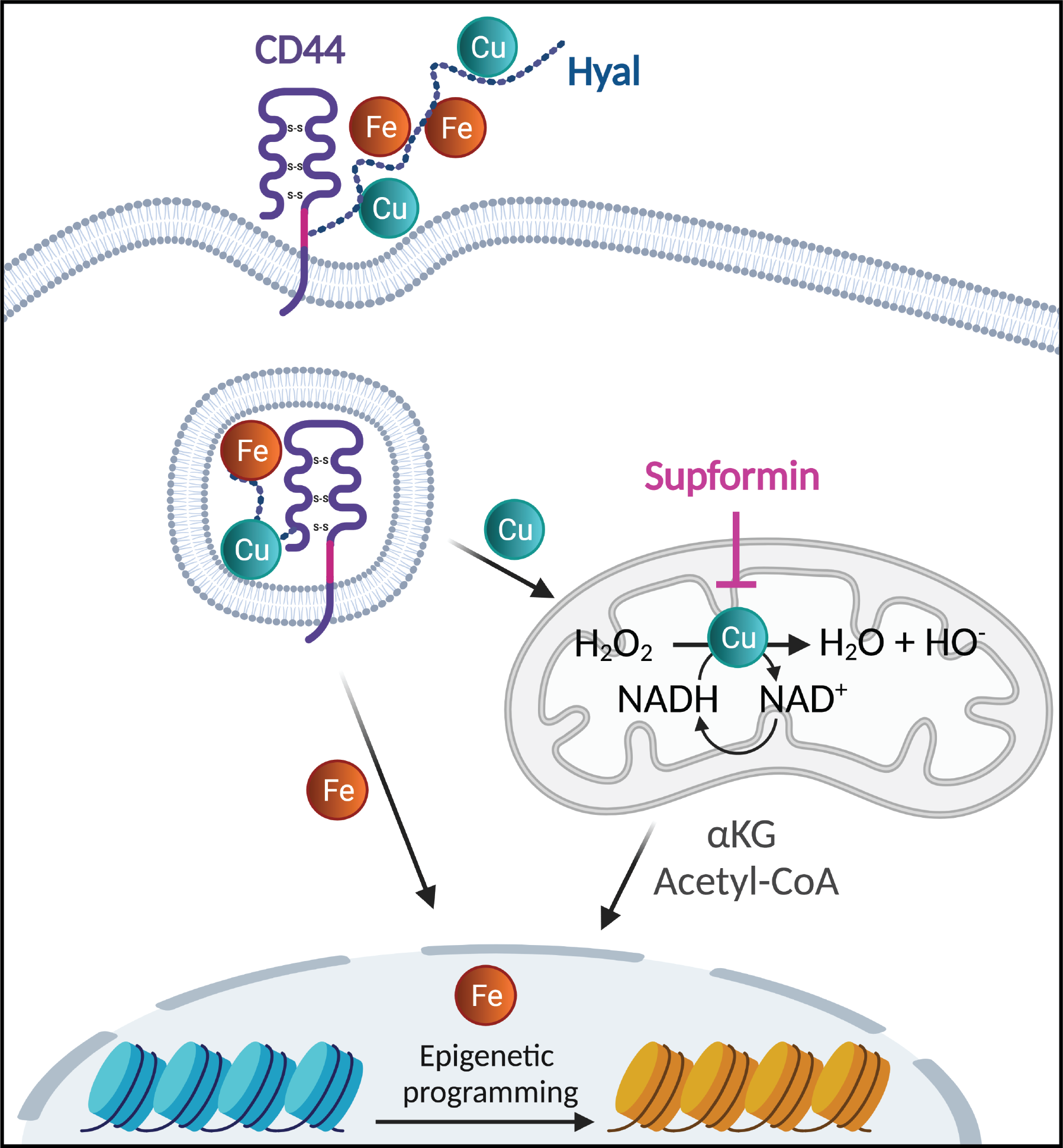
Our previous work3 on persister cancer cells made us wonder if CD44 could play a role in metal import in other cell types and regulate cell plasticity in a wide spectrum of cells. We set out to study this process in macrophages derived from primary monocytes isolated from blood. Using this system, we found that during inflammatory macrophage activation, cells predominantly take up metals via CD44, including copper, iron, calcium and magnesium4. Interestingly, canonical copper transporters had a marginal effect on copper uptake.
Where does copper go and what does it do?
Copper taken up by CD44 accumulates in mitochondria of cells, where it catalyses the interconversion of NAD(H) in a reaction using hydrogen peroxide. This might be of particular interest to the chemistry community, as one of the core reactions in biology, implicating a crucial and ubiquitous metabolite, requires copper in its +2 oxidation state (Cu(II)), a reaction that had already been described in 1980 but largely overlooked in the literature5.
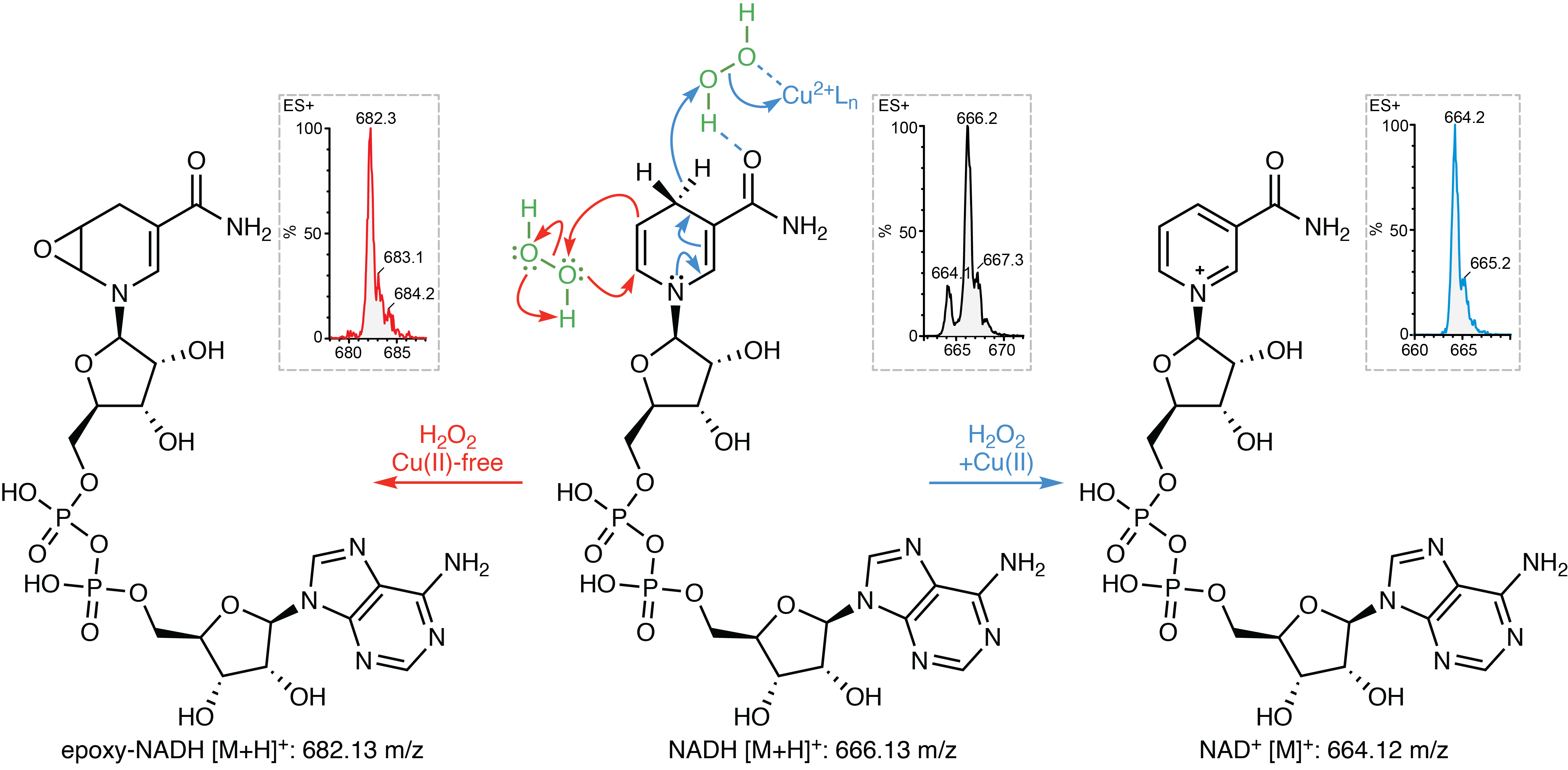
We investigated these processes further and screened for small molecules that could interfere with copper and macrophage cell plasticity. Using this methodology we identified metformin to have an effect on these processes. Indeed, metformin was reported back in 1929 to form complexes with Cu(II)6 and we previously observed that it accumulates in mitochondria7. Since metformin forms a 2:1 complex with Cu(II) we designed a new molecule - we later termed supformin - which links two biguanides together with a linker. This changes the inherent entropic effect and in fine has a potency 5000 more elevated than metformin in interfering with cell plasticity.
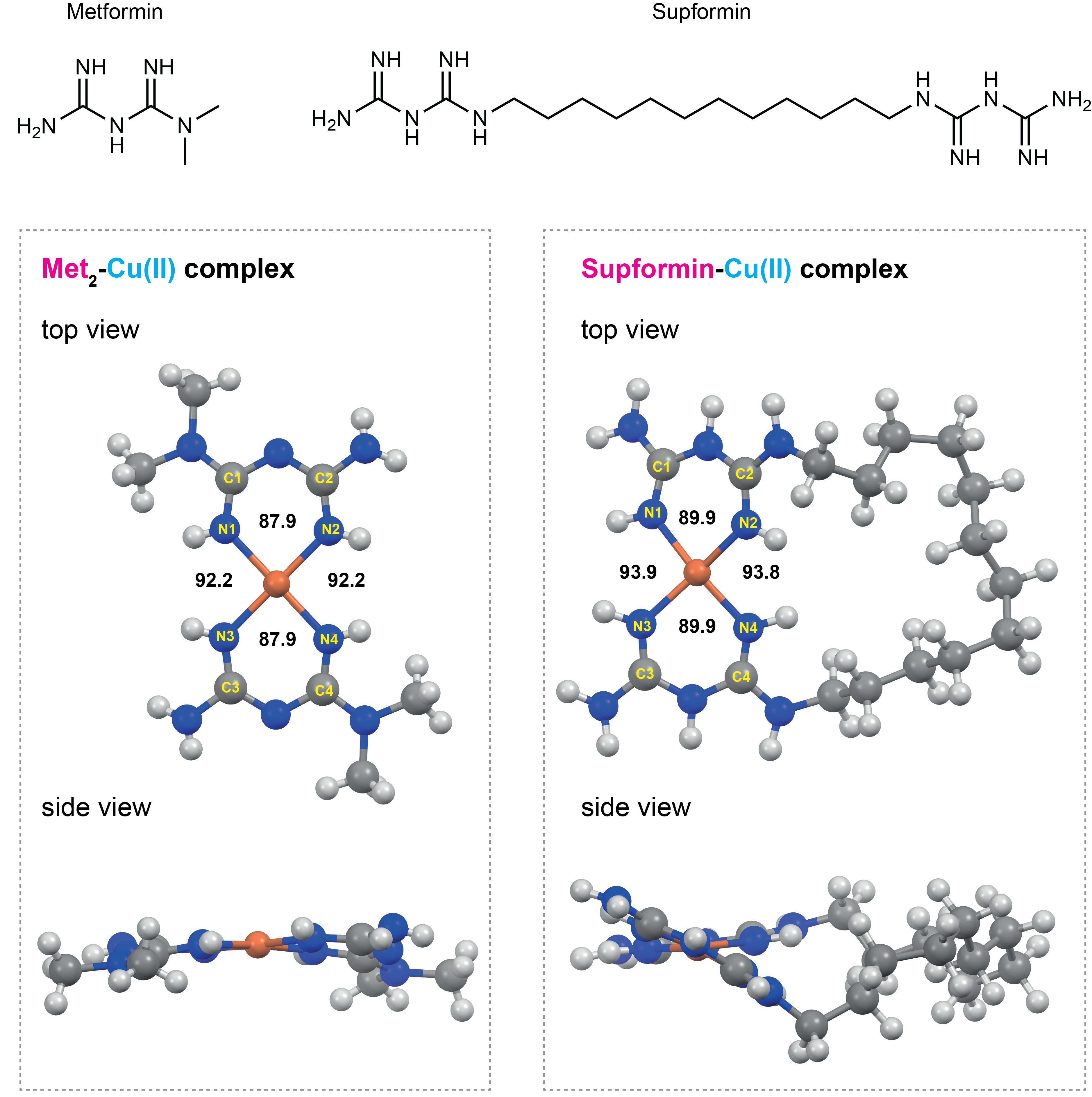
What are the effects of mitochondrial copper chelation?
We found that supformin can reprogram cell metabolism in macrophages and reduce total NAD(H) levels. Since these metabolites are crucial for many metabolic processes, including the Krebs cycle, we observed altered levels of several metabolites, of which acetyl-CoA and alpha-ketoglutarate stood out. These two metabolites are crucial for histone demethylation and acetylation in the cell nucleus, thus, changing their levels will ultimately lead to changes in the epigenetic landscape of the cell. Changes of the epigenetic landscape are inherent with changes in cell phenotype. Indeed, supformin treatment lead to epigenetic reprogramming into a less inflammatory state.
Finally, this was also observed in animal models of sepsis, where we could rescue survival of mice.
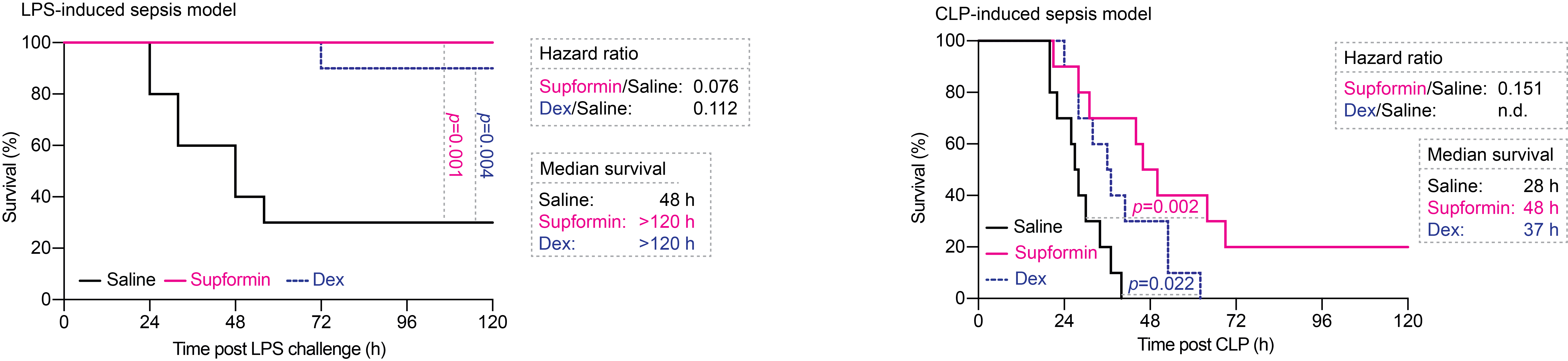
This work is, in our opinion, a good example of how chemical biology and the understanding of chemical reactivity can lead to major discoveries in cell biology.
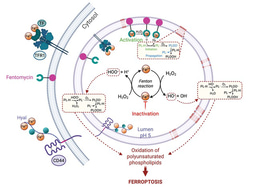
CD44 marks cancer stem cells rich in iron
Iron metabolism and homeostasis has been studied in a variety of settings, including immune cells and of course cancer. In a recent study, CD44 was shown to mark specifically iron-rich cells8, whereas TfR1-rich cells failed to do so. Thus, CD44 can be used to identify persister cancer cells that are rich in iron. This study also showed that these cells can be targeted specifically with small molecules that exploit lysosomal iron biology, to initiate lipid degradation in these organelles.
References
1 Cell. 1990 Jun 29;61(7):1303-13. doi: 10.1016/0092-8674(90)90694-a
2 J Cell Sci. 1993 Sep;106 ( Pt 1):365-75. doi: 10.1242/jcs.106.1.365
3 Nat Chem. 2020 Oct;12(10):929-938. doi: 10.1038/s41557-020-0513-5
4 Nature. 2023 May;617(7960):386-394. doi: 10.1038/s41586-023-06017-4
5 Biol Trace Elem Res. 1980 Sep;2(3):159-74. doi: 10.1007/BF02785352
6 Ber. dt. chem. Ges. 1929, 1390–1398. doi: 10.1002/cber.19290620604
7 PLoS One. 2018 Nov 6;13(11):e0206764. doi: 10.1371/journal.pone.0206764
8 Nature, 2025, doi: 10.1038/s41586-025-08974-4
Reference: Solier et al., A druggable copper-signalling pathway that drives inflammation, Nature, 2023, 617, 386–394, doi:10.1038/s41586-023-06017-4
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in