Challenging Thermodynamics: Combining Immiscible Elements in a Single-phase Nano-ceramic
Published in Materials
The Hume-Rothery rules governing solid-state miscibility limit the compositional space for new inorganic material discovery. Here, we report a non-equilibrium, one-step, and scalable flame synthesis method to overcome thermodynamic limits and incorporate immiscible elements into single phase ceramic nanoshells.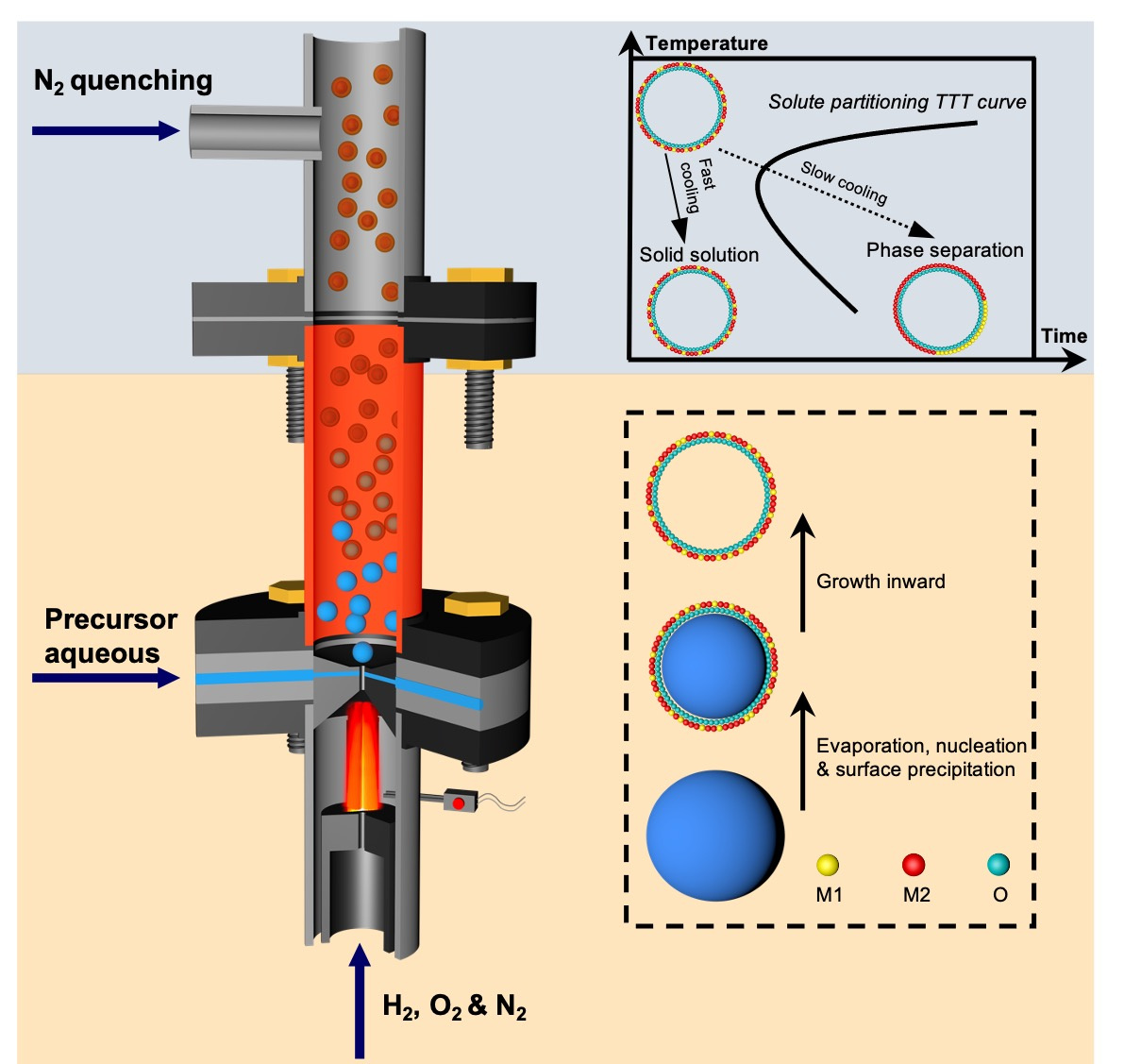
Figure 1. Schematic of the non-equilibrium flame aerosol process producing ceramic solid solution nanoshells via an evaporation driven droplet-to-particle conversion followed by N2 quenching. M1 and M2 represent metal elements used in aqueous inorganic salt solutions as precursors.
What is the key breakthrough of this research by the team from LBNL and University at Buffalo SUNY?
A: The key breakthrough is a universal non-equilibrium flame aerosol synthesis method that can create metastable ceramic solid solution nanoshells (See Figure 1). This method overcomes traditional limitations arising from elemental miscibility, allowing production of otherwise inaccessible materials with novel properties.
By integrating diverse pairs of metal oxides, the research establishes a general methodology to circumvent thermodynamic immiscibility, thereby enabling the creation of a broad array of homogeneous metastable solid solution nano-ceramics. These materials exhibit exploitable features such as highly dispersed solute atoms and high defect density, which can be tuned for specific applications. This approach opens up new opportunities for rational design of material properties for targeted applications, such as catalytic processes, including the efficient CO2 (dry) reforming of methane.
What makes the flame aerosol synthesis method innovative compared to traditional materials synthesis methods?
A: Traditional methods are often constrained by thermodynamic barriers, preventing the formation of certain compounds. The far-from-equilibrium flame aerosol method circumvents these barriers, enabling the production of ceramic solid solutions that are immiscible under near-equilibrium conditions. This innovative approach opens up new material spaces. Starting from prototype examples including (NiMg)O, (NiAl)Ox, and (NiZr)Ox, we went on to extend this method to a broad range of Ni-containing ceramic solid solutions, and finally to general binary combinations of elements as oxides.
Moreover, the materials synthesized using this method exhibit distinctive properties like hollow morphology, high defect density, and homogeneous elemental distribution. These properties are highly advantageous for applications in catalysis, sensing, and energy storage, among others.
What is the "encapsulated exsolution" phenomenon discovered in this study?
A: In general, exsolution is the process of a solute separating out of a metastable solid solution or compound. For example, nickel exsolution from Ni0.1Mg0.9O under reducing conditions produces nickel nanoparticles supported on MgO, which is an effective catalyst for dry reforming of methane. The "encapsulated exsolution" phenomenon is a novel behavior observed when heating a porous (Ni0.07Al0.93)Ox solid solution in a reducing atmosphere. This process forms Ni nanoparticles within the pores of the porous alumina shell rather than on its surface. This has several advantages over conventional exsolution, including fast exsolution speed, the formation of ultrasmall nanoparticles, and ultrahigh thermal stability. This could have significant implications for catalysis, as it allows for highly dispersed active sites and provides a nano-confined structure that resists sintering.
What are the implications of this research for industrial applications?
A: The scalability, low cost, and efficiency of the flame aerosol process make it an ideal candidate for commercial production. A prototypical material reported here has shown exceptional performance as a catalyst for CO2 reforming of methane, suggesting potential for industrial use based on its extraordinary combination of stability and activity. The ability to maintain catalytic activity over long periods without deactivation at high temperatures is particularly important for industrial processes. While we presented a specific example, metal nanoparticles supported on metal oxides are the most common form of heterogeneous catalyst, and the method of producing them introduced here should be broadly applicable to catalysts for a vast array of reactions of industrial importance.
Who led the research?
A: The research was led by a PhD candidate Mr. Shuo Liu as the first author, under the joint supervision of Dr. Chaochao Dun, Dr. Jeffrey J. Urban, and Dr. Mark T. Swihart. It represents a collaboration between the Lawrence Berkeley National Laboratory and the University at Buffalo, SUNY. This joint effort between LBNL and University at Buffalo SUNY signifies a significant advance in the rational design of materials.
For more details, please check out our paper "Challenging Thermodynamics: Combining Immiscible Elements in a Single-phase Nano-ceramic" (https://doi.org/10.1038/s41467-024-45413-w)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in