
In recent years the Liddle group has successfully prepared and characterised a variety of novel uranium-ligand linkages, mainly centred around group 15 elements. In particular, with a family of uranium-nitrides secured our attention moved to thorium since we believe the only way to gain true insight into the actinides is to be able to make comparisons between more than one metal in similar coordination environments. Thus, we thus attempted the synthesis of a thorium-nitride. In the Nature Communications paper this blog is about, we didn’t manage to isolate a thorium-nitride, but we gathered evidence for a transient nitride, synthesised some other cool molecules, and made some interesting observations that might have broader implications for our understanding of early actinide chemical bonding.
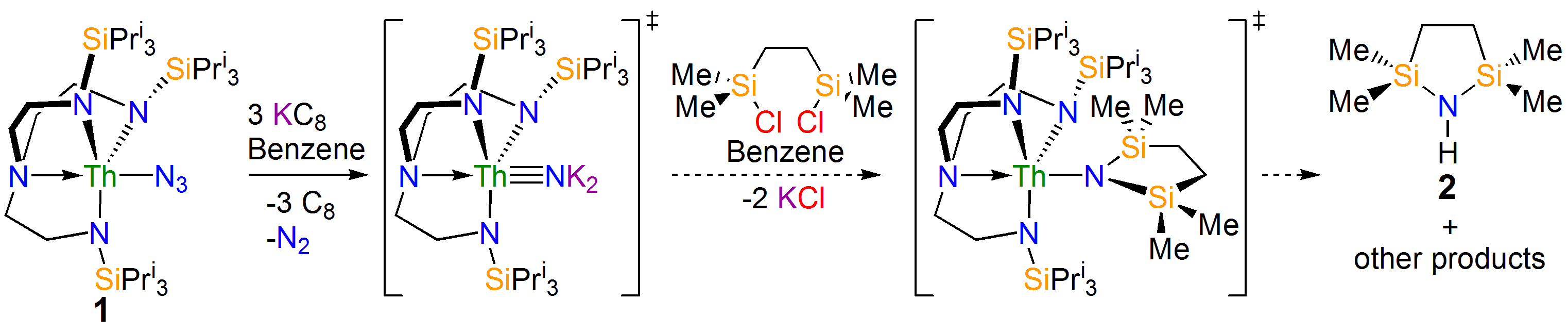
Our study began with the synthesis of the thorium-azide 1 as shown in the scheme above. When this compound is reduced in benzene dinitrogen is clearly evolved, but despite all our attempts we could not isolate a nitride. However, we could treat the intermediate and eventually isolate the nitrogen heterocycle 2 providing evidence of a transient nitride and nitride group transfer.
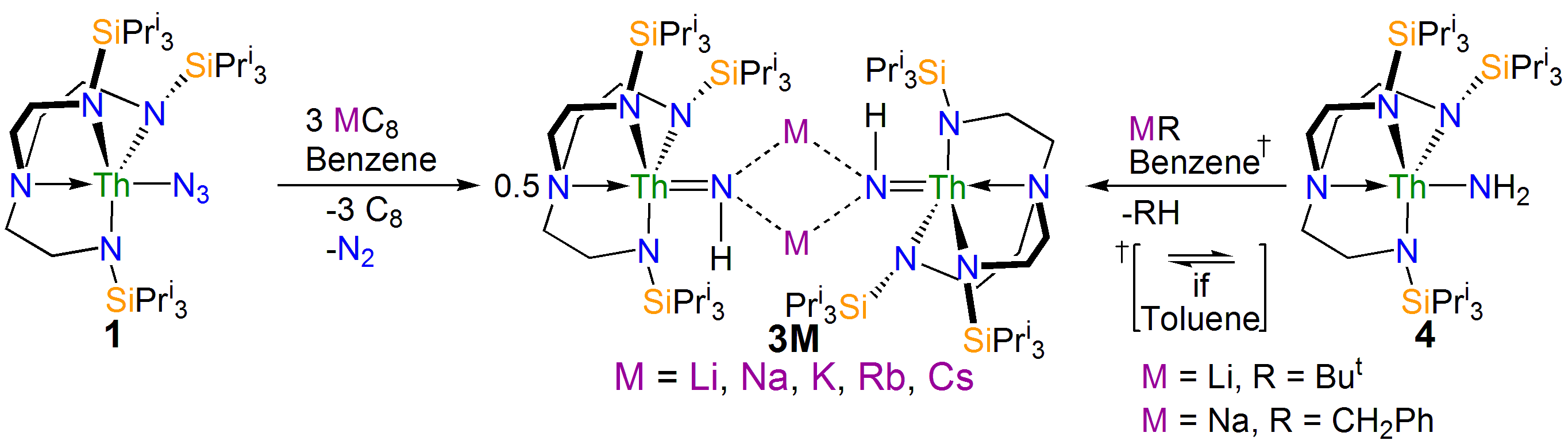
Interestingly, if the trapping agent to make the heterocycle is not added the reduction of 1 eventually results in isolation of parent imido complexes 3M as shown in the scheme above, where the imido nitrogen is derived from a transient nitride and the imido hydrogen is picked up from solvent. Furthermore, the parent imido dimers 3M can be accessed from the parent amide 4, but only cleanly in benzene; when toluene is used an equilibrium is established showing that these parent imidos can deprotonate toluene. This was in a sense frustrating, but in collaboration with Laurent Maron at the University of Toulouse we were able to computationally model the reaction profiles, and they clearly show the formation of reactive nitrides that are able to deprotonate benzene and toluene solvents to make the observed imido complexes, but the less basic imidos can only deprotonate toluene. This is gratifyingly entirely consistent with out experimental observations.
As we delved further into the bonding of these transient nitrides and indeed some of the parent imido complexes we made an unexpected discovery, namely that the s-bonds of these thorium-nitrogen multiple bonds are actually higher in energy than the p-components. This has been seen in calculations on uranium-nitrides and the uranyl dication, but was not expected for thorium. The origins of this are complex, but boil down to thorium 6p-orbitals being involved in the bonding, and when they are the normal energy ordering of p > s gets overturned. This is called pushing-from-below and whilst it has been invoked in uranium chemistry for many years it was long ago ruled out for thorium.
The observations of our work suggest that thorium too can partake in pushing-from-below. The question naturally arises: “so why hasn’t this been seen before?” Our working hypothesis is that nitrides are amongst the hardest/strongest donor ligands around, and perhaps this phenomenon only becomes apparent when in the ‘extreme’ bonding situations that nitrides promote. What also emerges, is that pushing from below seems to ‘kick-in’ more easily for uranium than thorium, which starts to suggest that this is a general but periodic occurrence. However, we suspect that this is just the beginning of this story, and certainly more work is needed to put this proposal onto a firmer footing. One thing is for sure however, thorium, quite pleasingly, is proving to be just as interesting and mysterious as its near neighbour uranium!
Blue links
https://www.nature.com/articles/ncomms13773
https://www.nature.com/articles/s41467-019-12206-5
https://www.nature.com/articles/nchem.1642
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in