Collection Highlight: Fibrosis, Cancer, and SDG3
Published in Biomedical Research

 Journal of Translational Medicine is an open-access multidisciplinary journal that serves as a bridge to optimize communication between basic and clinical science. As a platform from bench-to-bedside research, it publishes in specialized sections relevant to the Sustainable Development Goal 3 (SDG3): Good Health and Wellbeing, set by the United Nations.
Journal of Translational Medicine is an open-access multidisciplinary journal that serves as a bridge to optimize communication between basic and clinical science. As a platform from bench-to-bedside research, it publishes in specialized sections relevant to the Sustainable Development Goal 3 (SDG3): Good Health and Wellbeing, set by the United Nations.
A key target of SDG3 is to reduce premature mortality from non-communicable diseases (target 3.4), specifically in relation to mortality rates attributed to cardiovascular disease, cancer, diabetes or chronic respiratory disease (3.4.1) by 2030.
Journal of Translational Medicine contributes to the global efforts of researchers and clinicians to meet this target by publishing high-quality articles across several expert-led sections such as Cancer Microenvironment, Combination Strategies, Immune RadioBiology, and Translational Cancer Biology.
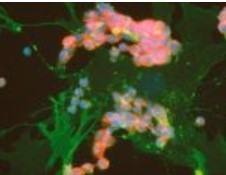
of carcinoma cancer cells of the lung.
Normal lung fibroblast cells are green.
Smaller carcinoma cells (red) are seen
growing upon this fibroblast lung tissue.
Both cell types have blue nuclei.
In 2023, the launch of the Fibrosis section, focusing on the normal and pathogenetic processes of tissue repair, led to the Fibrosis and Cancer Intersection (FACI) article collection. With research implicating fibrosis in the proliferation and migration of cancer cells, the collection was timely and has garnered over 40,000 article accesses.
Guest Editors Dr Mary Salvatore (Vice Chair of Education for Department of Radiology at Columbia University) and Dr Monica Pernia (Columbia University) expressed their objective for the collection in an Editorial:
“The improved understanding of how fibrosis develops, causes morbidity, and promotes cancer is the foundation for making advances in diagnosis, treatment, and ultimately prevention.”
This year's theme for World Cancer Day (4th February), "United by unique", highlights how every experience with cancer is unique. Hence, the FACI article collection is particularly topical in demonstrating this through the discussion of different types of cancers (breast cancer, lung cancer, ovarian cancer, pancreatic cancer, and prostate cancer), the systems affected (thoracic, abdominal, and genitourinary organs), and the treatments involved (immunotherapy, radiotherapy and other therapeutic interventions).

Dr Salvatore (Left) and Dr Pernia (Right)
In line with SDG3 target 3.4, the Guest Editors explain that a collection like Fibrosis and Cancer Intersection which is aimed at “increasing the understanding of the relationship between fibrosis and cancer development would be of great benefit for the medical scientific community since this knowledge could accelerate the development of new therapies to treat cancer while increasing the hostility of the cancer cells’ microenvironment by preventing or interfering with collagen deposition. This evidence could explain the development of tumoral resistance to immune defenses and existing or emergent anti-cancer therapies.”
We invite you to check out the Fibrosis and Cancer Intersection article collection!
Sign up for article alerts from Journal of Translational Medicine.
Follow the Topic
-
Journal of Translational Medicine

Journal of Translational Medicine is an open access journal publishing articles focusing on information derived from human experimentation so as to optimise the communication between basic and clinical science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Computational Modelling and Network Medicine in Drug Toxicology and Clinical Pharmacovigilance
Computational approaches are becoming indispensable in the field of translational pharmacology and systems medicine. In particular, the integration of computational modelling and network medicine is reshaping our understanding of drug toxicity, safety profiling, and clinical pharmacovigilance. With the rapid accumulation of multi-omics, electronic health records, and real-world pharmacovigilance data, there is an urgent need for advanced computational frameworks to identify hidden drug–target–phenotype associations and elucidate mechanisms underlying adverse drug reactions (ADRs) and toxicities. Drug toxicology and pharmacovigilance are key components of translational medicine, bridging molecular mechanisms with patient outcomes. However, predicting and preventing drug-induced toxicities remain challenging due to the complexity of biological systems and individual heterogeneity. Network-based and machine learning methods, such as graph neural networks, causal inference, and mechanistic modelling, are providing new opportunities to reveal system-level toxicity pathways, optimize drug safety assessment, and enhance early signal detection in clinical practice.
This collection aims to gather latest research and comprehensive reviews that explore computational, network-based, and AI-driven strategies in drug toxicology and pharmacovigilance. We seek contributions that demonstrate how integrative modelling, network pharmacology, and multi-scale data analysis can advance the prediction, understanding, and management of drug-related adverse effects, ultimately contributing to safer and more effective therapeutics.
Potential topics include, but are not limited to:
- Computational modelling for drug toxicity prediction
- Network medicine approaches to drug safety and toxicity mechanisms
-Machine learning and AI in pharmacovigilance signal detection
-Integrative modelling of drug–target–pathway interactions
-Systems pharmacology and multi-omics network analysis in toxicology
-Network-based biomarker discovery for adverse drug reactions
-Data-driven prediction of idiosyncratic and immune-related toxicities
-Translational applications of network and computational models in clinical drug safety assessment
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Sep 07, 2026
Harnessing Innovative Machine Learning Techniques to Combat Drug Resistance in Solid Tumors
In recent years, the challenge of drug resistance in solid tumors has emerged as a significant barrier to effective cancer treatment, often leading to treatment failure and poor patient outcomes. As traditional therapeutic approaches struggle to overcome this hurdle, there is a growing interest in leveraging innovative machine learning techniques to enhance our understanding of drug resistance mechanisms and improve therapeutic strategies. This article collection aims to gather cutting-edge research that explores the intersection of machine learning and oncology, focusing on how advanced algorithms can be applied to predict drug resistance, optimize treatment plans, and ultimately foster personalized medicine approaches in cancer care. By fostering interdisciplinary collaboration and sharing novel insights, this collection seeks to pave the way for transformative advancements in the fight against cancer.
This article collection explores integrating advanced machine learning methodologies—including ensemble learning, deep learning, reinforcement learning, explainable AI, and quantum computing—in understanding and overcoming drug resistance in solid tumors. We invite contributions highlighting novel algorithms, examples of innovative machine learning technique implementation, and interdisciplinary approaches, ultimately leading to improved therapeutic outcomes and enhanced patient care in oncology.
opics of Interest
The collection welcomes original research, reviews, commentary and methodology articles on the following topics:
• Ensemble Learning Techniques;
• Applications in predicting drug resistance profiles;
• Comparative studies of ensemble methods versus traditional approaches;
• Deep Learning in Oncology;
• Neural network architectures for tumor characterization;
• Image analysis and interpretation for drug response prediction;
• Reinforcement Learning;
• Adaptive treatment strategies using reinforcement learning;
• Simulation models for drug resistance evolution;
• Explainable AI;
• Methods for interpreting machine learning models in clinical settings;
• The role of explainability in improving treatment outcomes;
• Quantum Computing Applications;
• Quantum algorithms for optimizing drug discovery processes;
• Examples of quantum-enhanced machine learning techniques in oncology.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
This collection supports and amplifies research related to SDG 3: Good Health and Well-Being.
Publishing Model: Open Access
Deadline: Nov 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in