All life relies on water and, increasingly, drought disrupts life in many parts of the world. Of the severe consequences caused by drought, the decrease in agricultural yield is the most harmful to human civilization. Plant response to drought is well studied, but not the microbes that grow in and on plants. These microbes also respond to drought and their responses may also affect plant tolerance to drought.
In 2016, we conducted a drought experiment on a semiarid sorghum plantation in California1. Our three treatments are 1) a regularly watered control treatment, 2) natural, pre-flowering drought for the first nine weeks of growth followed by regular watering, and 3) natural, post-flowering drought treatment beginning after 10 weeks of regular watering. We sampled sorghum leaf, root, rhizosphere and soil, weekly, over the 17-week sorghum growing season.
By employing high-throughput, internal transcribed spacer (ITS) amplicon sequencing, we characterized the fungal communities of these samples. We found that the total fungal community developed along with the plant, that assembly of the fungal community shifted from processes that are stochasticity-dominated to those that are determinism-dominated, and that assembly was delayed by drought stress 2. We then focused on the most important group of beneficial plant symbionts, arbuscular mycorrhizal (AM) fungi, whose mutualism with plants is seen in the oldest land plant fossils and is nearly ubiquitous among modern plants. We found a rapid succession in the AM fungal community from species with R (ruderal) to C (competitive) strategies 3. This succession was coupled with shifts in chemical signal communication between the plant and AM fungi, as inferred from transcription of plant genes responsible for synthesis of strigolactone and reception of lipochitooligosaccharide 4. Using the same plant and soil samples, our colleagues investigated bacterial communities using 16S amplicon sequencing. Their results showed that drought delays development of the sorghum root microbiome and enriches for monoderm bacteria 5, and that iron metabolism plays an important role in the dynamics of drought-induced rhizosphere bacterial microbiome 6.
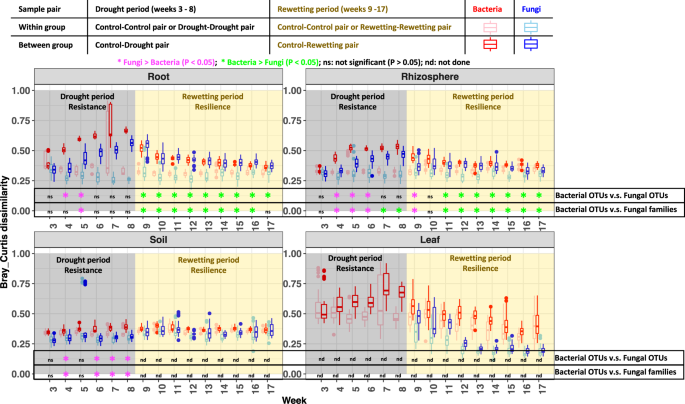
However, a key gap remains about the difference in drought responses between bacterial and fungi. Guided by the simple fact that fungi grow more slowly than bacteria, it had been assumed that fungi, as compared to bacteria, are (i) more resistant to drought stress but (ii) less resilient when rewetting relieves the stress 7. We subjected these hypotheses to analyses at the levels of community composition and microbial associations. We found, using community composition, that fungi, as compared to bacteria, are (i) more resistant to drought stress but (ii) less resilient when rewetting relieves the stress, qualifiedby stronger resilience of the fungal than bacterial community was observed in the first week of rewetting. Results were more complex using all-correlations and co-occurrence networks, where drought disrupts microbial networks based on significant positive correlations among bacteria, among fungi and between bacteria and fungi. Surprisingly, co-occurrence networks among functional guilds of rhizosphere fungi and leaf bacteria were strengthened by drought, and the same was seen for networks involving arbuscular mycorrhizal fungi in the rhizosphere. Our analyses enabled testing of the stress gradient hypothesis, which proposes that positive microbial associations should increase in frequency under stress scenarios, such as drought. The results supported the stress gradient hypothesis because drought increased the relative frequency of positive correlations. We also detected a number of network hubs and connectors that are taxa disproportionally important in structuring microbial communities.
Our discoveries about the complexity and specificity of co-occurrence networks of fungi and bacteria open new questions: i) do other stresses strengthen microbial networks, ii) what are the molecular and chemical mechanisms that strengthen microbial networks and, iii) can networks be used to mitigate drought stress in agriculture, perhaps by introduction of netwrotk hub taxa?
1 Varoquaux, N. et al.Transcriptomic analysis of field-droughted sorghum from seedling to maturity reveals biotic andmetabolic responses. Proc. Natl. Acad. Sci. U. S. A., 27124–27132 (2019).
2 Gao, C. et al. Fungal community assembly in drought-stressed sorghum shows stochasticity, selection, and universal ecological dynamics. Nat. Commun. 11, 34 (2020).
3 Gao, C. et al. Strong succession in arbuscular mycorrhizal fungal communities. ISME J. 13, 214-226 (2019).
4 Gao, C. et al. Successional adaptivestrategies revealed by correlating arbuscular mycorrhizal fungal abundance with host plant gene expression. Mol. Ecol., 10.1111/mec.16343 (2022).
5 Xu, L. et al. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc. Natl. Acad. Sci. U. S. A. 115, E4284-E4293 (2018).
6 Xu, L. et al. Genome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics. Nat Commun 12, 3209 (2021).
7 de Vries, F. & Shade, A. Controls on soil microbial community stability under climate change. Front. Microbiol. 4 (2013).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in