CRACCing the code of Effector T cells
Published in Cancer, Microbiology, and Cell & Molecular Biology
Explore the Research
SLAMF7 defines subsets of human effector CD8 T cells - Scientific Reports
Scientific Reports - SLAMF7 defines subsets of human effector CD8 T cells
Understanding CD8 T Cell Differentiation and Potential Therapies: A Breakthrough Study
CD8 T cells, the immune system’s natural defenders, play a pivotal role in combating infections and cancer. These cells differentiate into distinct subsets, each with specialized functions. However, their efficiency can decline due to aging or persistent viral infections, such as HIV, CMV, or EBV. A recent study explores the intricate differentiation pathways of CD8 T cells, revealing critical markers, transcriptional mechanisms, and therapeutic opportunities to reinvigorate immune responses.
Key Findings and Insights
- CD8 T Cell Diversity: Markers and Subsets
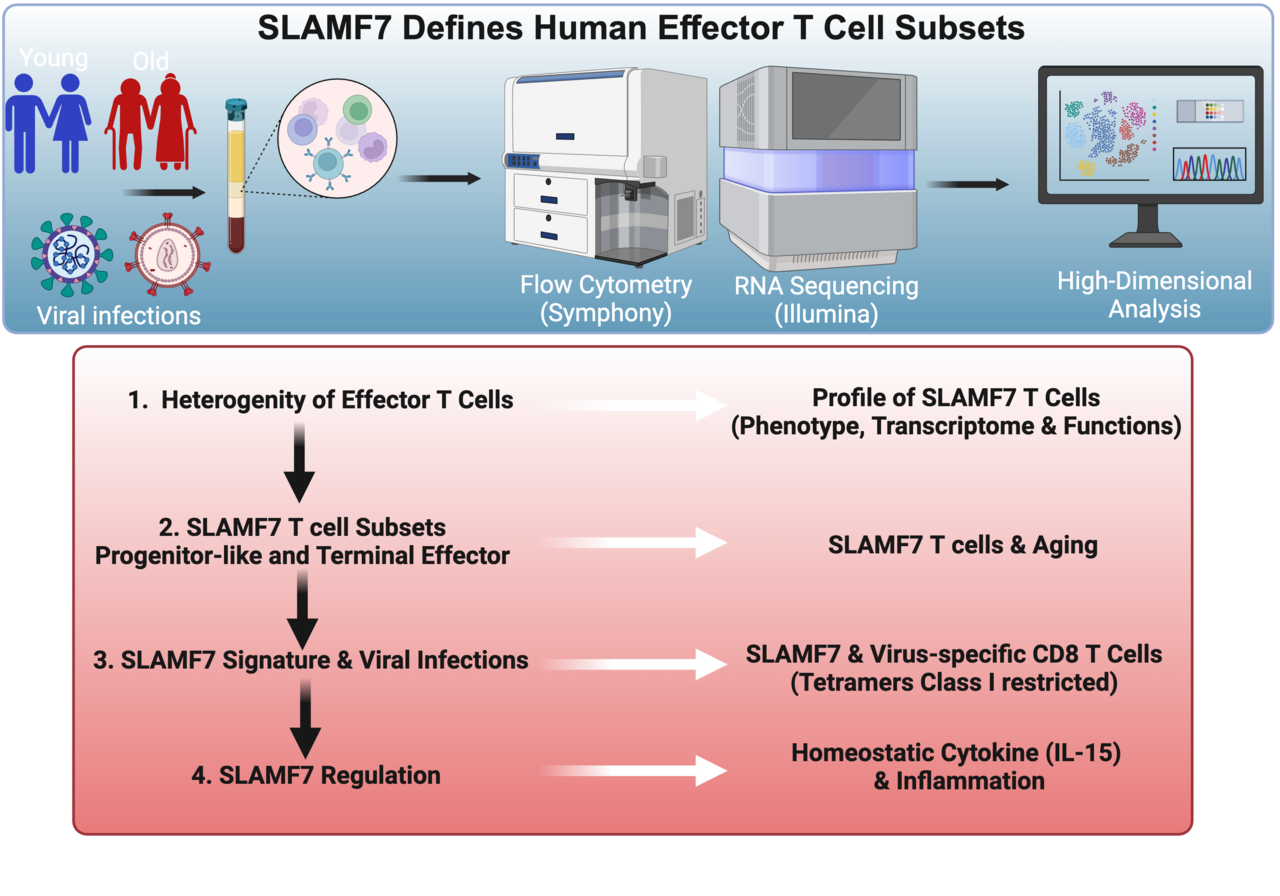
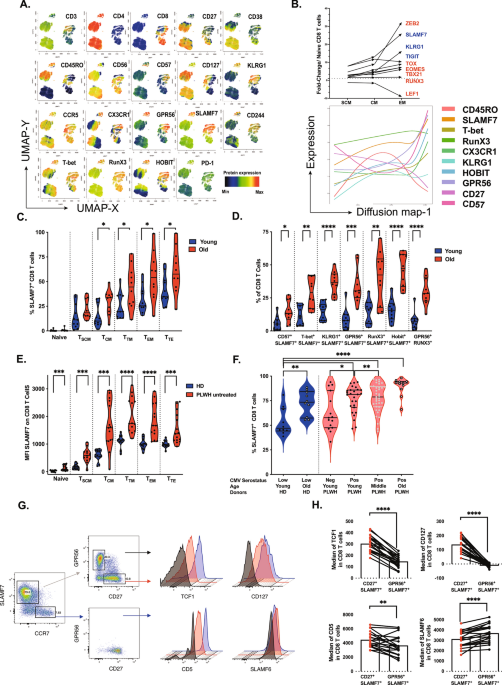
The study investigated the ontogeny of terminally differentiated CD8 T cells, focusing on surface markers like CD27, CD57, GPR56, KLRG1, and the relatively underexplored SLAMF7 (CRACC). These markers reflect how CD8 T cells evolve under various conditions, including aging and chronic viral infections.
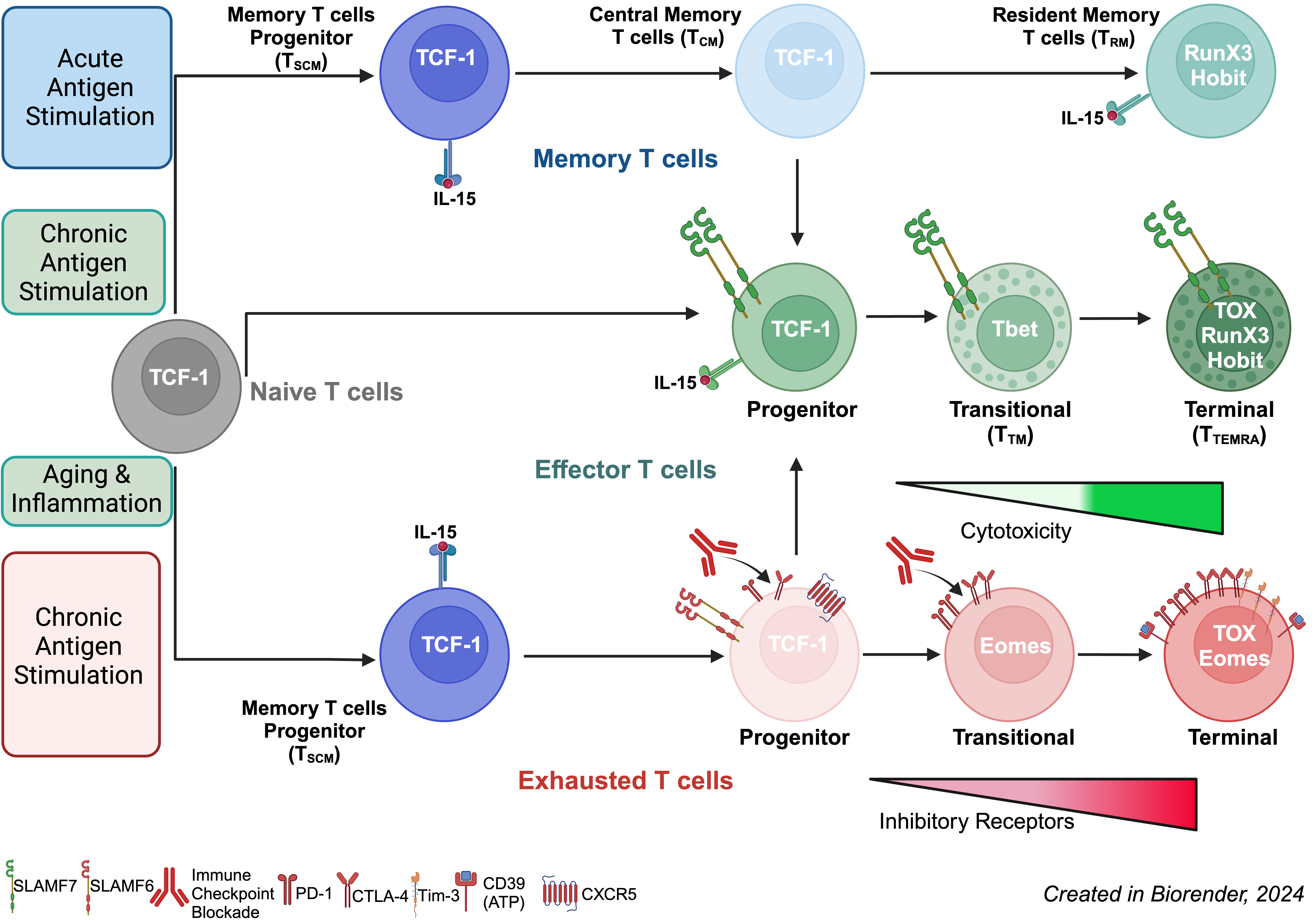
- Progenitor-like T cells: Early-stage cells with self-renewal capacity and the potential to differentiate into effector T cells.
- Effector T cells: Cytotoxic cells equipped with perforin and granzyme B to destroy infected or cancerous cells.
- Exhausted T cells: Prolonged antigen exposure as in chronic conditions, reduced functionality of these cells.
The team identified SLAMF7, CD27, and GPR56 as key markers to distinguish progenitor-like cells from terminal effector T cells, offering a refined classification system.
- SLAMF7: A Novel Marker in Differentiation
SLAMF7 was found to be acquired early during CD8 T cell differentiation, challenging the traditional notion of labeling CD8 T cells as “senescent” based solely on the loss of markers like CD27 or CD28.
Key Insights:
- SLAMF7 distinguishes early-stage progenitor-like T cells from terminally differentiated effector cells.
- SLAMF7+ T cells were enriched in chronic infections (e.g., HIV, CMV) and older individuals, reflecting the immune system’s response to persistent antigen stimulation.
- Therapeutically, targeting SLAMF7 could enhance immune surveillance in both infections and cancer.
- Transcription Factors: Unlocking CD8 T Cell Potential
The study also explored the transcription factors (TFs) that regulate CD8 T cell differentiation:
- TCF-1 and TOX: Exhausted T cells include subsets ranging from progenitor-like cells (TPEX) to terminal exhausted cells. TCF-1 determines the stemness of TPEX, enabling regeneration and effective response after immune checkpoint blockade therapies, (e.g., anti-PD-1 or anti-CTLA-4). In contrast, TOX acquisition is linked to multiple inhibitory receptors, proliferation/effector function loss, and metabolic changes.
- T-bet and Eomes: Effector T cells with a T-bethigh Eomeslow phenotype exhibit sustained anti-virus/tumor responses. This signature corresponds to terminally differentiated T cells, distinct from the exhaustion signature (T-betlow Eomeshigh).
- RunX3 and HOBIT: These TFs drive the terminal differentiation of CD8 T cells into a cytotoxic state and promote a tissue-resident memory program, essential for combating cancer and infected cells in the tumor microenvironment.
- Effects of Aging and Viral Infections
The study highlighted how aging and chronic viral infections reshape CD8 T cell differentiation:
- In older individuals, CMV-specific CD8 T cells exhibited elevated TOX levels and reduced TCF-1 expression, impairing their ability to control latent infections.
- Chronic viral infections (e.g., HIV, CMV) promoted differentiation toward terminal states but also led to exhaustion, marked by low TCF-1 and high TOX expression.
Therapeutic Implications
The findings present exciting opportunities to rejuvenate immune responses through targeted interventions:
SLAMF7-Targeted Therapies
- SLAMF7 engagement could enhance immune surveillance in chronic infections and cancer.
- Therapeutic approaches may involve boosting progenitor-like T cells or reprogramming exhausted cells into functional effectors, especially for immunotherapy-resistant cancers.
Leveraging IL-15
- IL-15 promotes the expansion of SLAMF7+ progenitor-like cells and supports long-term immune memory.
- In aging populations, IL-15 could rejuvenate CD8 T cell responses by boosting tissue-resident CD8 T cells and Stem-Cell-like Memory T cells (TSCM).
Reprogramming Exhausted Cells
- Modulating transcription factors like TCF-1 and TOX (or Tet-2) could restore the functionality of exhausted T cells, leading to stronger responses in cancer and infection therapies.
Future Directions
This study highlights critical advances in understanding CD8 T cell differentiation, particularly the roles of SLAMF7 and transcription factors. Key implications include:
- Using SLAMF7 as a biomarker to identify and enhance effector T cell subsets.
- Targeting transcriptional regulators like TOX and TCF-1 to rejuvenate exhausted T cells.
- Developing therapies that combat the immune-aging process and mitigate the effects of chronic infections.
While promising, these findings require further validation through preclinical models and clinical trials. The ultimate goal is to translate these insights into therapies that strengthen immunity in aging populations, patients with chronic viral infections, and individuals battling cancer.
Conclusion
This research underscores the complexities of CD8 T cell differentiation and their essential role in immunity. Identifying novel markers like SLAMF7 and exploring transcriptional dynamics, opens the door to therapies that enhance immune resilience. From boosting effector function to reprogramming exhausted cells, the future of immune restoration looks brighter than ever.
SLAMF7 Defines Subsets of Human Effector CD8 T Cells
T cell differentiation and Transcription Factors expression
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in