Deciphering how nature installs reactive warheads onto peptides
Published in Chemistry and Cell & Molecular Biology
Microorganisms are masters of synthesis, assembling complex molecules which they can deploy as weapons to compete with other organisms in their environment. Many of these natural molecular weapons possess reactive functionalities (warheads) which can irreversibly inhibit essential biochemical machinery to kill competing organisms in the environment. For many years, we have exploited microbial chemical warfare to develop medicines that can save lives. For instance, penicillin antibiotics are derived from microbial natural products containing a β-lactam warhead, which irreversibly inhibits transpeptidase enzymes that construct the cell wall – essential for the survival of bacteria. Peptide natural products armed with epoxide warheads also led to the development of Carfilzomib, a potent proteasome inhibitor used to treat cancer. Most recently, Nirmatrelvir, a peptide drug with a nitrile warhead which targets a protease essential for viral replication, was approved to combat COVID-19. The synthesis and optimisation of peptides with reactive warheads is challenging and currently relies on outdated and polluting chemical synthesis which can be problematic to implement on the scale required for drug development. To address this, we aimed to explore how nature installs warheads onto peptides.
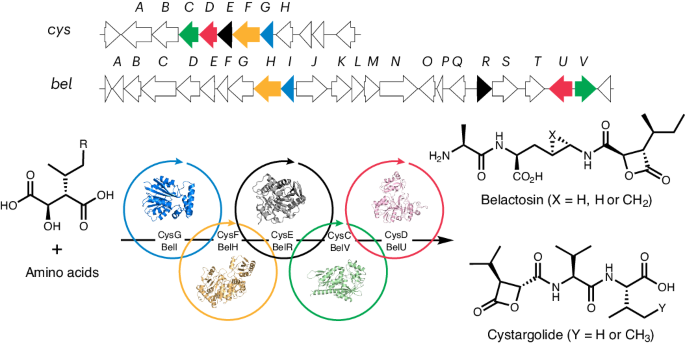
We focused on the pathways to cystargolide (cys) and belactosin (bel), which are natural peptides produced by bacteria. The cys and bel peptides possess β-lactone warheads which can cross-link and inhibit proteasomes, making them potent anticancer agents. The chemical synthesis of β-lactone containing peptides has been widely explored but is not straightforward. Typically, the synthesis of optimised cys and bel derivatives requires 10-12 steps with yields lower than 10%, using harsh reaction conditions and toxic reagents. The biosynthetic gene clusters (BGCs) encoding the enzymes predicted to assemble cys and bel were identified through in silico (bioinformatics) analysis (Figure 1a). Interestingly, analysis of the BGCs suggest that cys and bel are likely biosynthesised by discrete ligase enzymes, rather than ribosomes or a non-ribosomal peptide synthetase (NRPS), which are commonly found in the biosynthesis of other peptide natural products. Although there has been speculation on how nature assembles cys and bel, experimental knowledge about the enzymatic logic for biosynthesis of β-lactone containing peptides has remained elusive.
We produced and characterised all the essential enzymes required for cys and bel assembly, to reveal nature’s secret for making these peptides (Figure 1b). Surprisingly, we found that the β-lactone warheads of cys and bel were biosynthesised in a three-step sequence (1→2→3→4) involving cryptic methylation, rather than the more direct route that was predicted from in silico analysis. We also discovered that after assembling the warhead, distinct ligase enzymes sequentially add amino acids to the β-lactone acid to create the final peptide natural product. Intriguingly, all the enzyme functions we confirmed experimentally differ from the initial bioinformatics predictions.
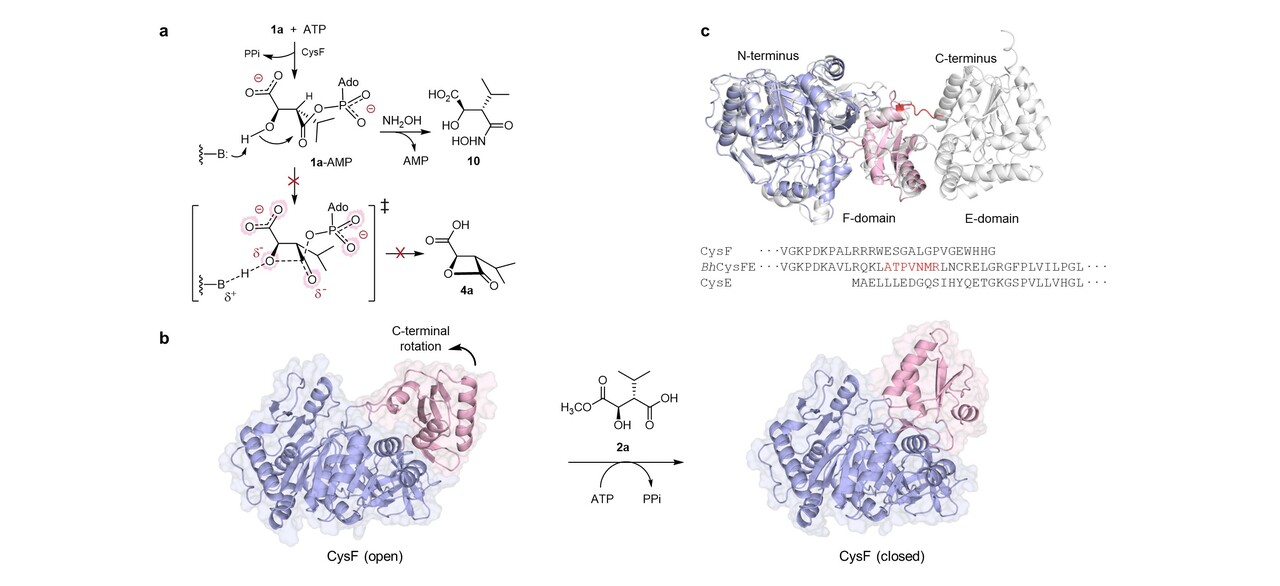
We were particularly interested to understand why nature employs three enzymes and two high energy cofactors, S-adenosyl methionine (SAM) and adenosine triphosphate (ATP), to construct the warhead. Typically, nature assembles molecules in a highly step-efficient manner. However, in cys and bel biosynthesis nature has evolved a highly unusual protection-cyclisation-deprotection route to assemble the reactive, strained four membered β-lactone ring. To explore this unusual and cryptic enzymatic logic, we solved the crystal structure of the key lactone synthetase CysF and showed that it resembles other acyl-adenylating (acyl-AMP forming) enzymes. Testing the unmethylated substrate (1) with CysF revealed that although the enzyme activates this substrate, formation of a C4-acyl adenylate cyclisation does not occur. Based on structural analysis, we suggest that electrostatic repulsion between the negatively charged C1-carboxylic acid and the phosphate group of the adenylate moiety, in the sterically demanding transition state, likely prohibits the direct cyclisation of precursor 1 to the lactone acid 4. To overcome the steric and electronic constraints, nature has evolved a methyltransferase (CysG) to temporarily block (protect) the C1-carboxylate, facilitating β-lactone formation (CysF), before promptly removing the methyl group (deprotection) post-lactonisation using a hydrolase (CysE).
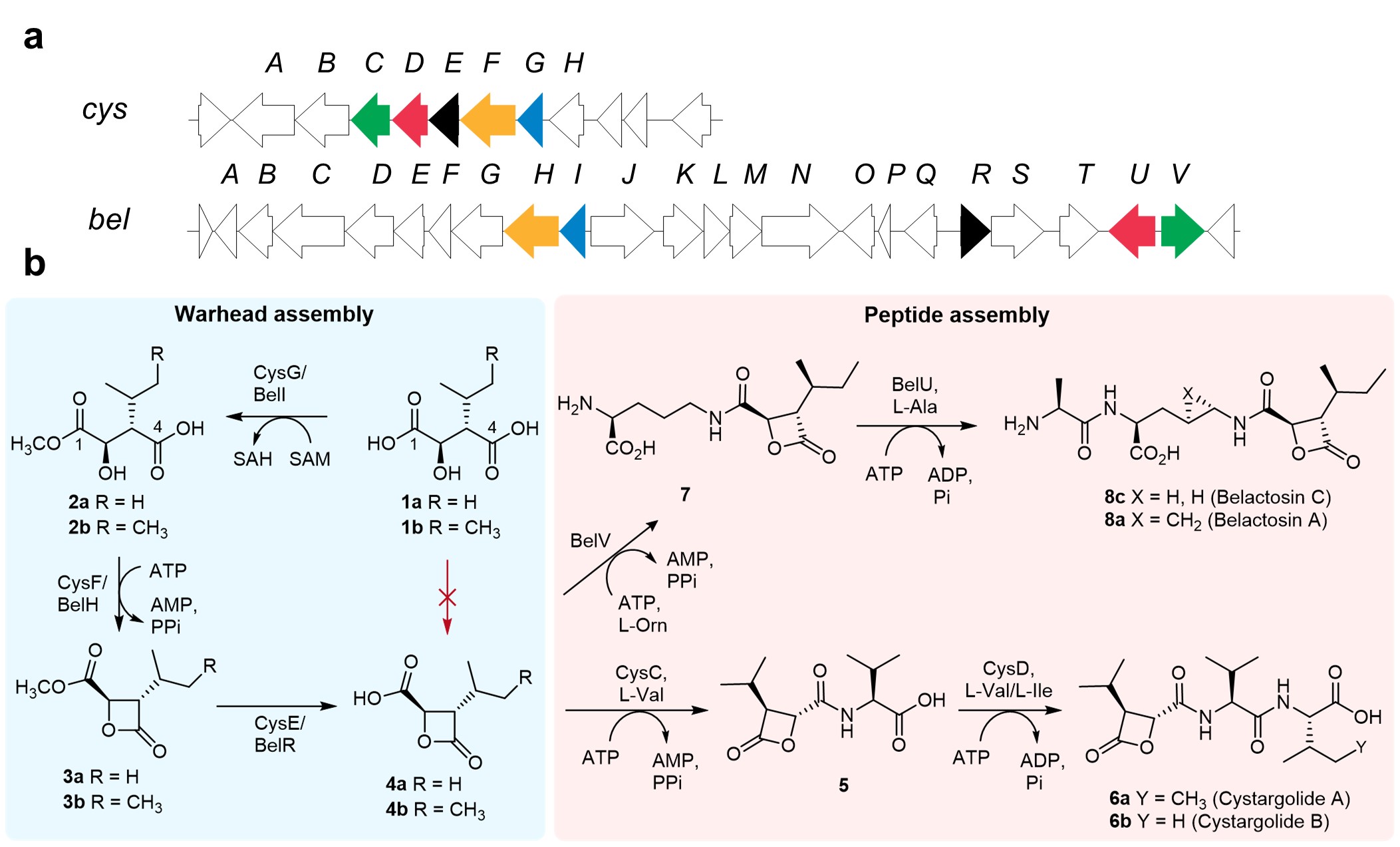
Figure 1: Solved biosynthesis of β-lactone-containing peptides and enzymatic synthesis of peptide therapeutics. a, Cys and Bel biosynthetic gene clusters. b, in vitro characterisation of Cys and Bel pathways.
Examining similar cys BGCs, we also found that in some bacterial species, the β-lactone synthetase CysF and the hydrolase CysE were fused into one bifunctional enzyme. We confirmed the activity of one of these CysFE bifunctional fusion enzymes and showed it converts the methylester 2 to the lactone acid 4 quantitatively. Structural modelling using Alphafold showed that the β-lactone synthetase and hydrolase domains of CysFE were linked together by a short peptide sequence. Moreover, the model reveals the possible existence of a tunnel between the two active sites in CysFE, which may serve to channel the unstable β-lactone methyl ester (3), protecting this reactive β-lactone from hydrolytic ring-opening in the aqueous media.
In addition to elucidating the entire pathway for the biosynthesis of cys, we also characterised each step in the bel pathway. We leveraged the flexibility of standalone ligase enzymes in both pathways to develop enzymatic cascade reactions, delivering over 40 β-lactone-containing peptides in a clean and efficient manner. Notably, we were able to produce cys and bel derivatives in a single cascade reaction in up to 64% yield, which is a significant improvement over the 10-12 step chemical synthesis routes (ca. 10% yield) currently used to produce these promising anticancer agents. The fundamental insights and methods we have developed could prove valuable for the future enzymatic synthesis of a wide range of peptide targets for therapeutic and other important applications.
This research has now been published in Nature Chemical Biology.
https://www.nature.com/articles/s41589-024-01657-7
For a summary of the article, see:
https://www.nature.com/articles/s41589-024-01658-6
Guangcai Xu, Daniele Torri, Sebastian Cuesta-Hoyos, Deepanjan Panda, Luke R. L. Yates, Rémi Zallot, Kehan Bian, Dongxu Jia, Andreea I. Iorgu, Colin Levy, Sarah A. Shepherd & Jason Micklefield
https://twitter.com/Micklefield_Lab
Follow the Topic
-
Nature Chemical Biology

An international monthly journal that provides a high-visibility forum for the chemical biology community, combining the scientific ideas and approaches of chemistry, biology and allied disciplines to understand and manipulate biological systems with molecular precision.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in