Cytoskeleton moves
Published in Protocols & Methods

For Nature Methods I recently did a story called Actin in action, which is about the ways labs work with cells to learn about cell function, in particular to track the cytoskeleton and its parts, such as actin. They combine methods, develop and use new trackers and think about the ones they wish they had.
Among others, I interviewed Nikki Reinemann from the University of Mississippi.
The cell works with a range of building blocks with a variety of properties, she says. Microtubules and actin filaments have different levels of stiffness; motor proteins have varying motility and facilitate different types cellular processes. Filament crosslinkers can be pliable in different ways and have various types of propensities to build hierarchical geometries. “These disparate Lego pieces must work together and communicate across large scales in order for robust cellular processes, such as motility and division, to take place,” says Nikki Reinemann.
Marileen Dogterom from Delft University of Technology.
“For mechanobiology, the combination of labels and sensors is very powerful,” says Marileen Dogterom.
Johns Hopkins University researcher Douglas Robinson:
His rule of thumb, says Douglas Robinson, is to use two or more types of reporter that look at the system from different angles. “Especially for actin, which is a highly allosteric polymer, I have never really trusted any single probe.”
Uri Manor from the Salk Institute:
“Overall, our understanding of how cytoskeletal mechanical forces affect organelles is very poor, which to me means it is very exciting to see so much progress in new probes and tools for studying these subcellular structures,” says Uri Manor.
Michael Sixt from the Institute of Science and Technology Austria, Roland Wedlich-Söldner from the University of Münster (see podcast below),
Basically, coming from two different backgrounds helped to cover more ground. And I was very aware of the actin field at that time, he had many years experience working on actin, and what issues would come up. And then there was this very simple thing that helped to spread it, we gave it to over 400 groups before it was published. So I was very active, in sending out the probe and also very detailed information about what to do and not to do, says Roland Wedlich-Söldner.
Ronald Rock from the University of Chicago:
LILAC opens the door to a number of imaging modes that take advantage of photoswitching, says Ronald Rock.
and Christophe Leterrier, a Centre National de la Recherche Scientifique (CNRS) researcher at the Institute of NeuroPhysiopathology, which is affiliated with CNRS and Aix Marseille University.
He kindly shared some beautiful micrographs and I decided to make a video with them. Here is the video Cytoskeleton and chill with micrographs and comments from Christophe Leterrier:
And I chatted with the developers of Lifeact, Michael Sixt and Roland Wedlich-Söldner about their label, the labels they use and the ones they wish for and a bundle more. Here is a podcast with them and a podcast transcript is pasted below.
You can listen to it here and it's also on the streaming podcasts such as Apple podcasts, Google podcasts and Spotify.
Note: These podcasts are produced to be heard. If you can, please tune in. Transcripts are generated using speech recognition software and there’s a human editor. But a transcript may contain errors. Please check the corresponding audio before quoting.
Transcript of The push-pull of cells
Michael Sixt
Most immunologists study cellular locomotion and cell shape, because they want to understand how this contributes to the immune response. So in my lab, it's a bit different, we are kind of turning it around. So we are rather using the immune cell as a more or the immune system as a model system to understand single cell behavior. So I'm personally more interested in cells than in whole organisms. So my, the animal, I look at is the cell usually.
Roland Wedlich-Söldner
An interesting direction for me was a bit opposite from Michael where he started maybe from medicine and came more into the fundamentals of biology. I started off as a very basic geneticist, and then into cell biology through my first mentor, Gero Steinberg, who was really very good light microscopy, experts.
And then when GFP was invented, I basically got just hooked on the on the imaging part. And so that basically, just seeing visually, what happens in a sub cellular context live was something that's so fascinating to me that I got stuck with it with this kind of approach.
Vivien
That was researcher Michael Sixt from Institute of Science and Technology Austria. And next was Roland Wedlich-Söldner from the University of Münster in Germany. Hi and welcome to Conversations with scientists, I'm Vivien Marx. Today's episode is about the forces theatcells exert on one another and the forces acting inside cells.
If scientists want to watch cells in action, they use a microscope. If they want to track dynamics, especially the forces the tugging, pushing and pulling that goes on in cells, the mechanodynamics, researchers need microscopes and labels.
The cytoskeleton is sometimes compared to a railroad track network in a cell. It connects point a to b and all through the alphabet. But it's unlike a railroad track and more, say like the staircases in Hogwarts that have ways of moving about often when people were on them.
When the cytoskeleton moves around, it's usually not to play pranks but to get serious work in cells done. So it itself moves and it moves all sorts of things around the cell.
It's useful to have labels that can help researchers follow that movement.
I heard about a new label called LILAC from the lab of Ron Rock at the University of Chicago. The lab developed a version of the label Lifeact that is optogenetic. A link to that study is in the show notes and I interviewed Ron Rock and other researchers for a story I did on the cytoskeleton. A link to that piece called Actin in action is also in the show notes.
Actin in Action
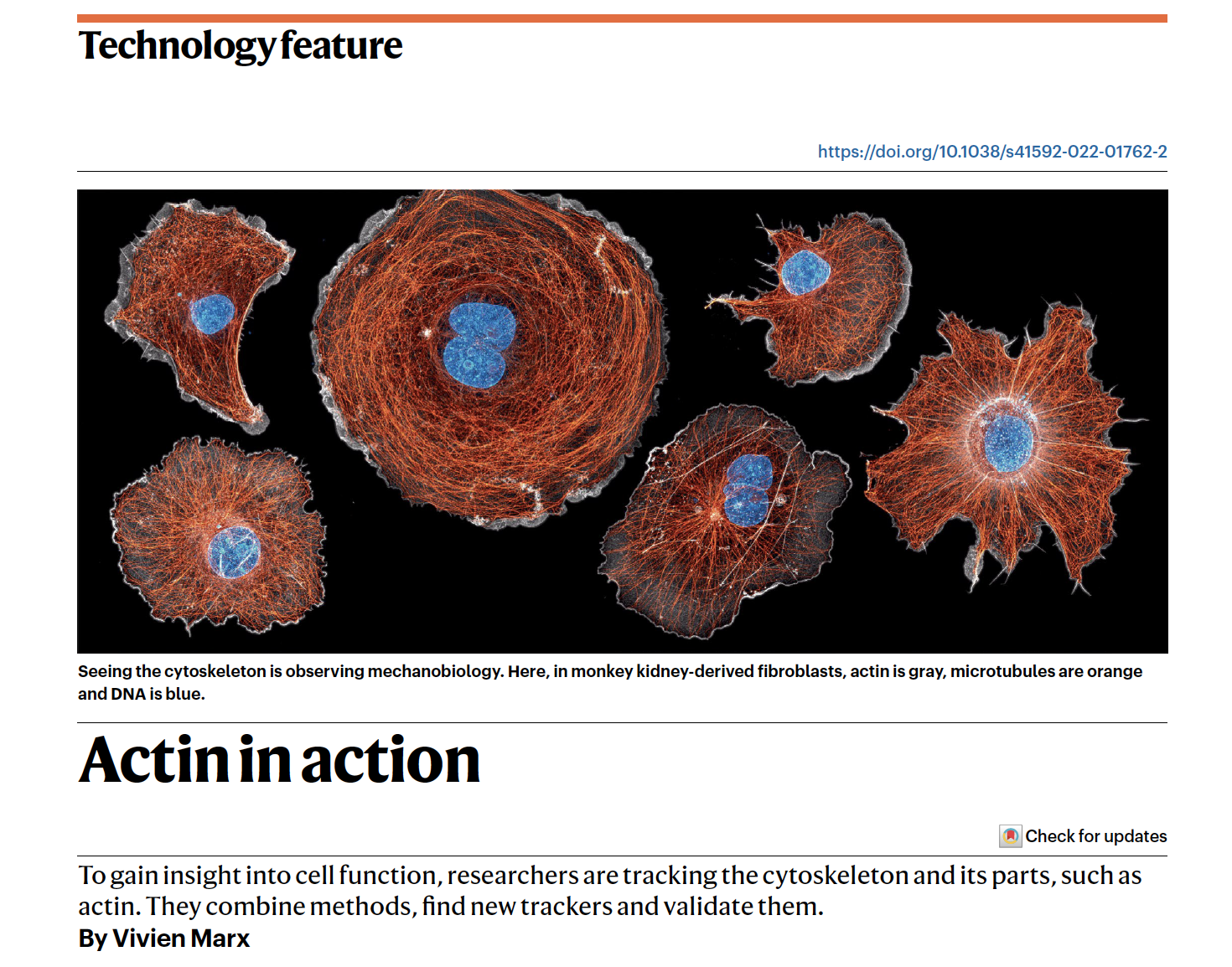
https://www.nature.com/articles/s41592-022-01762-2
LILAC: enhanced actin imaging with an optogenetic Lifeact

https://www.nature.com/articles/s41592-022-01761-3
As I read about LILAC I wondered about its parent so to speak, Lifeact, an established label for live-cell imaging, it labels filamentous actin, which is part of the cytoskeleton.
So I thought I would seek out the developers of Lifeact to find out a bit more about how it came to be and to hear what they thought about LILAC and to get their perspective on labels they use and the ones they would like to see developed. And it was also a good opportunity to ask more generally about the mechanical forces in cells.
Lifeact is popular, widely used and at the same time, in some hands Lifeact does seem to cause ungreat things as Ron Rock and colleagues point out in their LILAC paper. It can cause shape changes to mesenchymal stem cells. And when used in fruit flies it can cause sterility in fruit flies Hmm. And in a little bit I will share with you what the Lifeact developers said about that.
Lifeact is used in labs to perform live-cell imaging of the actin cytoskeleton. It was presented to the scientific world in a paper in 2008.
https://www.nature.com/articles/nmeth.1220
LIfeact: a versatile marker to visualize F-actin
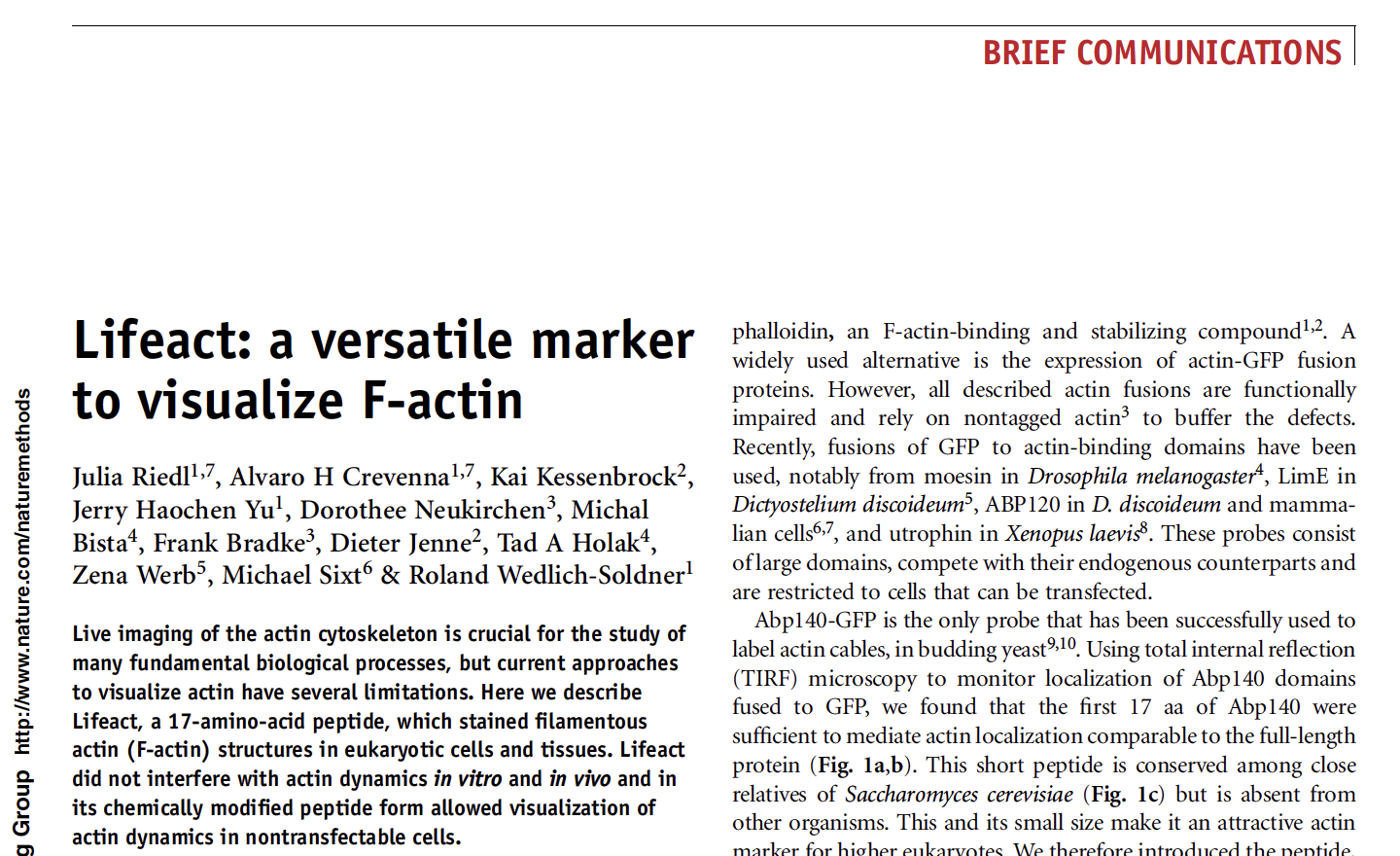
But actually the developers had been sending it around and you will hear more about that shortly. Lifeact was born from of a collaboration between several scientists Michael Sixt and Roland Wedlich-Söldner and their colleagues. And different fields of study came together and you will hear about that shortly, too.
At the time, Michael Sixt was a junior group leader at the Max Planck Institute of Biochemistry in Martinsried near Munich. And he is now at the Institute of Science and Technology Austria. Roland Wedlich-Söldner was then also a junior group leader at Max Planck Institute of Biochemistry and he is now at University of Münster in Germany
Michael Sixt
We were next door at the Max Planck.
Vivien
That's Michael Sixt. And I asked him and Roland Wedlich-Söldner about their background and the collaboration mechanics. Micheal Sixt was a physician and Roland Wedlich-Söldner was a yeast geneticist. But they developed other scientific interests also due to the interaction they had.
Michael Sixt [4:25]
I actually, I started off as more as an immunologist. So I'm a medical doctor. And then I started to work on immune cells. But I'm interested in immunology. But then I became more and more interested in how the single cell works, actually.
Most immunologists study cellular locomotion and cell shape, because they want to understand how this contributes to the immune response. So in my lab, it's a bit different, we are kind of turning it around. So we are rather using the immune cell as a more or the immune system as a model system to understand single cell behavior. So I'm personally more interested in cells than in whole organisms. So my, the animal, I look at is the cell usually, and this is also it started off a bit different, but it was also the influence that I had, including Roland, in, in Martinsried when I had my first Junior group, where things became more and more basic. And so now we are really looking at basic mechanisms of how cells change, shape and migrate.
Vivien
Here is Roland Wedlich-Söldner who also mentions Zena Werb who was part of Lifeact development, too. That's Zena Werb from the the University of California San Francisco who was spending some time at the Max Planck Institute.
Michael Sixt
We were next door at the Max Planck
Roland Wedlich-Söldner [5:45]
Including also the one of the other authors was just visiting on a sabbatical Zena Werb who was also on the Lifeact paper. She was basically in our Institute for that year, on and off for a year. So it was a beautiful, very interactive environment. An interesting direction for me was a bit opposite from Michael where he started maybe from medicine and came more into the fundamentals of biology. I started off as a very basic geneticist, and then into cell biology through my first mentor, Gero Steinberg, who was really very good light microscopy, experts.
And then when GFP was invented, I basically got just hooked on the on the imaging part. And so that basically, just seeing visually, what happens in a sub cellular context live was something that's so fascinating to me that I got stuck with it with this kind of approach.
So not just a general cell biologist, but I would say that I'm very much driven by microscopy approaches, basically. And what that means is that I'm not only I don't care so much about the organism, but I also don't care about the particular cell type that I'm looking at what I really care about is how fundamental concepts or mechanisms of organization happen on in biology.
And that happens on all scales, so they can be in an organ in a tissue or in a single cell. But very often, this boils down to self organization. So you don't have a nice hierarchy that geneticists tried to teach me initially, we have a nice regulator that goes down in the linear pathway and single outcome, But you always have networks. And there's something that got us has tracked with these systems biology, or mathematicians that you might have seen in the in the CV.
Because to understand network behavior, nonlinear interactions, you need to get some some grasp of these concepts in math and physics that would say in theoretical physics. So this basically colored a little bit the direction of our research.
And with Lifeact basically got me from a purely yeast focused and micro organism focused research more into the mammalian side. And also, more recently in the medical aspect, because we now had a marker that basically was much more relevant for many people working on mammalian cells. So I think this became feasible because we basically covered that aspect. And broaden my horizons during the time and Martinsried a lot.
Vivien
Lifeact is for looking at actin and is part of a general area of science called mechanobiology, which looks at the forces for example the forces acting on cells. I wondered about the force in interactions such as when actin and myosin interact in a cell. Or when an immune cell goes after an invader, is it gentle interaction, or is it kind of cellular bodyslam. Here's Michael Sixt.
Michael Sixt [8.25]
Interesting question. Yeah. I like that. When, so what is definitely true is like when, when, like in the immune system, there are always cells meeting and they make the synapses, the strands and synapses. And we actually looked a bit at the synapses. And it's not that. So it's really an exchange of mechanical forces also. So we saw actually, that in the synapses, the antigen-presenting cells and the lymphocytes and they constantly push into each other. So once one pushes in, that triggers a reaction and the other one, and the other one pushes back, and then it goes back and forth. And this is how they're kind of massaging each other and, and building a platform for the receptors to exchange information.
And so let's say, especially immune cells, they're relatively weak they do not do major deformations, of the of the connective tissue, which would also be a problem, right? So if you have a fibroblast, the fibroblast, it really pulls on the environment, and it, it contracts it and puts tensile forces on the extracellular matrix, whereas the immune cells, they just go through and don't want to change a lot. Because they constantly migrate through the body. And, and if they would change a lot, that would cause potentially a lot of damage that would have to be repaired. So it depends on the cell type. But I would say, a fibroblast that can really hold on its environment, that that are significant forces. And this also makes then the, the structure of, let's say, an interstitium, that it's really under tension. And, and, and this builds the stability of the connective tissue. It depends on the cell type. But in principle, it's a muscular system, right? That, that works in muscles that works on a, in a single cell.
Roland Wedlich-Söldner
I think one one part to add, so one is the amount of force you to generate, which can be significant is even single cells, but especially if you have then multiple cells acting together. The other thing is how fast this force acts. So when you asked about this, bam, like a micro-organism smashing into a cell, there, you have to consider that basically on this on the scale where micro-organism operates, the density of its environment is extremely high. So it rarely goes through air or through free liquid, but it goes through a very gelatinous, very viscous elastic environment. So it never has this option of really becoming very fast. So this is a type of bacteria, it's often called the swimming behavior in low Reynolds number, I think that's a physical term, where basically they go through goo right, through honey. So speed they reach is fairly low, even if they exert quite significant forces, and the same and more would be true for viruses, of course, COVID. And I think it's, in my case, I've been exposed to that a lot by except actually a journalist, right. So my wife wrote a book about slime as the hydrogel is basically surrounding everything.
Vivien
which is a great topic. Great topic.
Roland Wedlich-Söldner
Yeah, she's also a scientific journalist. So I know basically, your side of the job as well. But this basically shows in all aspects of biology that these kind of viscoelastic hydrogels that are either in the matrix and the surrounding are also in the cytosol, or the cytoskeleton is another example. Beautiful example. It's an active viscoelastic substance.
They basically drive a lot of the mechanical side and how these interaction work. That also makes perfect sense is going back and forth pushing behavior because you have these kind of elastic elements tween that connect everything.
So you probably never have this this simple pushing into each other might have a few examples like a bacterium with a comet tail, like Shigella, or Listeria that have comet tails of actin that they hijack from the cell. And they really propel themselves like little rockets through the cell, and they zoom around like crazy. This is the one time where I really saw fast speeds and mechanics, probably. They look like rockets. Really, when we imaged them with the lifeact, for example, you can see these comet tales as beautifully.
Michael Sixt
What is not really intuitive is how the interstitium looks like or how the tissue looks like for the cell. Because on the one hand, yes, if you put yourself into the shoes of a cell, then the the fluid medium around it is more or less like honey.
So it is really when the cell moves, and it stops the motor, then it stops instantaneously, because there is no inertia. But so this is one thing, but then if you look at a cell in the tissue, then things are actually, again, totally different.
Because there is actually almost no free water in the tissue. Everything, all the water is bound to sugars, right. And the sugars are actually under-saturated with water.
So if you measure the atmospheric pressure in the skin, in example, it's sub atmospheric. So there is suction. And so there is no free water moving around. And only if you have an edema, then if you push into the skin, then it leaves a dimple behind because then you can push away water, but in a normal skin, you cannot push a dimple. Because all the water is bound to sugars, so how it actually looks like the biophysical environment of a cell within a real tissue, we don't understand that too well, actually. And we also lack the the tools to mimic it in vitro, I think this is really a challenge to reconstitute the realistic tissue in in vitro and then look at the cell behavior.
Roland Wedlich-Söldner
One thing to add on this because it's a very recent thing with the Nobel Prize for Carolyn Bertozzi, who partly might be helping to solve this issue because he basically established click chemistry approaches to modulate this glycocalyx and extracellular matrix components that are usually very difficult to manipulate for a biologist because they're not genetically encoded. So they're basically very complex sugar molecule. And this is basically the last frontier of biology we can manipulate proteins, DNA, lipids, to some extent, but sugar the last thing that are the most difficult because they're the most complex and the least easy to manipulate and genetic level. But her techniques allow that, and there are also groups in Germany and worldwide approaching this. So hopefully to create a much more realistic environment, both on the cell surface, the glycocalyx. And in the extracellular matrix with hydrogels. There are groups here for example, who basically make very defined matrices that you can then manipulate by by light or by chemistry, to change intensity, ligand exposure, and so on.
Vivien
That was Roland Wedlich-Söldner and before him was Michael Sixt. Clearly there are a lot of aspects to explore about the mechanobiology of the cellular environment and Lifeact, the actin label plays a role in that. I wondered as they both look back to when they developed Lifeact what motivated them to do so. There was, it seems a lack of ways to study the cytoskeleton. Here's Roland Wedlich-Söldner on that aspect.
Roland Wedlich-Söldner [15.50]
I can say a few things maybe maybe I can add. So the most important thing is we didn't plan this, we didn't have any way prediction that this would be so important. And to some extent, you would expect, it's self organizing, like in the cell as well. So there are a couple of factors that came together. And that made it so successful. And I think, if you would plan it again, it probably won't happen. I think it's also a lot of randomness associated with it there was clearly a lack of optimal marker, there's still a lack of optical optimal, optimal probe for this because life act as other markers as well, it's not completely without its effect. And that's part of the basis for all of us for the new paper, is that are some defects associated with Lifeact.
But the key was that all the known probes that were around had very well documented phenotypes, so you cannot really use them for very sensitive processes in the cell. And they were quite spread out over different systems. And some people used one market in plants, another use them in yeast, in mammalian cells, you had two or three others, there was no uniform approach, and the level of detail of our knowledge about these markers were also very different.
So people did not spend the time to really characterize the basic properties well, and I think that was one of the key ingredients of the Lifeact paper, we characterized really on various levels going from biochemistry to cellular systems going from from different, even whole organism and then different cell types, characterize the key properties from of Lifeact in very much detail.
So people were quite confident that a lot of the question that they would have asked, are already answered. So basically, coming from two different backgrounds helped to cover more ground. And I was very aware of the actin field at that time, he had many years experience working on actin, and what issues would come up. And then there was this very simple thing that helped to spread it, we gave it to over 400 groups before it was published. So I was very, in sending out the probe and also very detailed information about what to do and not to do.
So I gave them a list, including all the problems that are associated with the Lifeact, right up front, so I didn't try to hide that. And I think that helped a lot to get it accepted. And because I knew people in the field also, I probably sent it to two key figures already that then propagated it further.
Vivien
I wondered who the first users were, people working on yeast, plants or people across the board working on eukaryotic cells.
Roland Wedlich-Söldner [18.25]
So my background was with both plant and fungi, bacteria as well, to some extent, Michael from immunology, which is a very important field for this migration. And mammalian cells well, It's really everyone working on that. So it's not one field, I would say. But we covered nearly everything. Later on, people tried to use it in more than malaria and parasitic organisms where it became a bit more limited. But that's one thing that we didn't cover. But in terms of community, people that I meet on meetings, I think that this covered nearly everyone working on actin.
Michael Sixt
I think really, before the paper was out, there were already all kinds of organisms with expressing Lifeact back there, because because Roland was very proactive in sending it out. And I think that make really a difference. Because the more people use it, the more experiences out and the more the lower the bar is for others to use it. And then it kind of becomes self organizing, right?
Roland Wedlich-Söldner
Then that also might help help in this case is that life is so short that many people can simply put it on a primer to make their favorite construct. You don't even need to send out the plasmid. You can basically tell them this is the sequence, put it in wherever you want. So this I think, was not not a not an unimportant point in spreading it.
Vivien
Lifeact is a peptide, 17 amino acids long or rather I should say 17 amino acids short. The peptide is fused to GFP, green fluorescent protein and as a label it highlights filamentous actin in eukaryotic cells and tissues. Perhaps Lifeact can be made even shorter. Here's Roland Wedlich-Söldner
Roland Wedlich-Söldner
And we're actually working on this right now. And we can cut it down to 11. And that still works. So we can make it much shorter. And it's still a very good probe.
Vivien
Probes can always affect systems and with Lifeact I came across some issues, at least in some labs that for example it changes the shape of stem cells when it's used and it can cause sterility in fruit flies when used in those animals. Ron Rock and his team at the University of Chicago developed an optogenetic version of Lifact called LILAC. That doesn't mean Lifeact will no longer be used even if it is an older marker.
Roland Wedlich-Söldner [20.35]
But the short point there is, although you say it's an old marker, there is no better marker at the moment, then like that. So and also with Rockäs s paper, there will be no better markers. So the new paper will give a new option, but it's not a replacement for Lifeact by far. So I don't see any way that it can play that role. It's too complex for that.
All these defects that are listed somewhere, in the very few cases to have been clearly attributed to Lifeact directly. So in many cases, it's simply overexpressing. The marker, sometimes they could clearly show it's only the GFP part does that. Sometimes it's so complex that by expressing something in a cell, you perturb the balance and lead to some defects that are completely unrelated to the actin marker. In some cases that did show basically, only with the Lifeact itself, you have the defect, but then you might have added a few control experiments to lower expression and gotten around that problem.
So it's clear from the beginning, which was also in the letter that I sent, everyone if you overexpress massively Lifeact, you absolutely misorganize actin, and you kill every cell.
So that's very easy to achieve if you have too much of it. So it was always clear that you need to find the right amount, so that you can still see it, but you don't disturb actin.
And if you make stable cell lines, for example, for mammalian cells, this is automatically done by the cell because they will somehow adapt so that they can propagate and divide. So you don't need to do all this the selection, you can do FACS analysis, or FACS sorting, if you want to really use a very defined population.
But the simple fact that we can make a whole life hack mouse that expresses life at nearly every single cell type, that is absolutely happy, there was no obvious phenotype, right. So we didn't go into all the details of behavior. But there was no phenotype whatsoever, that means they can completely live happily without, with a certain amount of Lifeact.
Michael Sixt
And it's still the only mouse that survives it. So if you do like, if you do actin GFP mice here we directly couple g-actin into GFP, and they, they are very, very dim view, it's very difficult to detect. Because simply if you would express it higher the animals die. So to my knowledge, the Lifeact mouse is the only one that that is reporting actin and survives it right.
We once got a very small mouse. It was extremely green. But it's also if you just express GFP and make a homozygous, then then they also don't survive it. So but of course, yeah, it's it's a reporter, right? So it's like every every approach to measure something has its side effects. So one, you have to know your system. And you really have to know what, what you want to observe, in order to control for the for the possible artifacts you're generating. I think there is no general rule been one can apply here.
Vivien
When using Lifeact Michael Sixt has some general advice.
Michael Sixt [23.50]
Do the right controls in your experiment? Of course. That's the message.
Roland
Yeah, very often the first figure in a paper would be repeating what has been done but exactly that's system that you're gonna use the rest of the paper so that you basically make sure that your baseline is all solid. Understood because of LifeAct, just having you mentioned that 1800 citations, but I think for the last 10 years or so people have stopped citing it because it's been so ubiquitous. So they're probably six to 7000 papers, including Lifeact. So you have all the information you could ever imagine around there. And I have seen actin groups without Lifeact at least partly using that in recent years anymore. So it's basically everywhere. He's also the amount of information available on it is basically far beyond one order of magnitude higher than anything else you have.
And not a single report in their show that there's a fundamental problem with having the Lifeact back in the beautiful, Most beautiful experiments are really the recent publications of the structure to choose papers and Cryo-EM that showed like a decorated actin filaments, where they show with high resolution and they to get the structure that needed to decorate filaments 100%, that means every single subunit of actin has a LifeAct bound to it, it's far more than you would ever get in a cell. And they showed it even with this saturation, you do not change actin structure at all, it's completely identical to the free actin filament. So that means that Lifeact doesn't do anything to act it, the only thing it does, it binds there, obviously.
And what they've also shown with many other similar structures is that nearly every actin binding protein goes to the same place, that there's one groove one spot on the actin surface, that seems to be the common attachment site for nearly every site binding protein. So there's crosslinkers, there's myosin, cofilin that turns over actin and Lifeact. Also toxins from bacteria that bind to this and beautiful in the structure study, they even show that Lifeact protect actin from these toxins, for example, which otherwise would kill the cell, because they compete for the same binding site. And then that's the big advantage of Lifeact. It's so weakened, its binding, that the affinity is so low that basically it does not prevent all these essential factors in the cell to bind.
So the main thing you have to play with is the affinity. There's probably no you to bind anywhere else, because otherwise nature would have done it. And you have dozens of different domains, they all go to the same place. For some reason, it's probably there's not nothing else available and actin because it's so densely packed into helix already.
Vivien
Then I asked them both about LILAC, the optogenetic version of Lifeact from the Rock lab. Here's Roland Wedlich-Söldner:
Roland Wedlich-Söldner [26.30]
So we both reviewed it, but I think I am, my time with it was not so long ago. And basically, in the end, I accepted it, obviously. So I like it. The main thing is, I think because Lifeact hack is so well established. And you have so much detailed information, including the structure now, it was possible to basically design rationally design this combination with with the or with a optogenetic tool.
But it's also a really cool idea to just bring to pretty short peptides that are very complex in their conformation together to make them really controlled in their interaction with with their ligands, respective ligands. So I wasn't expecting this to be so easy for Lifeact binds to a very dense surface on actin, there's not much space for it, to put this, this other peptide right next to it in a way that it can still be controlled, was to me already not intuitive, and I think quite, quite interesting that it works on principle.
Then obviously, because of these slight defects Lifeact has, you might want to monitor actin at some level during your experiments. And if you're looking at let's say more complex systems like organoids or tissues, you sometimes have to monitor them over weeks and months. So you might want to know at some point how they act and organize, but you don't want to constantly express Lifeact , because you would have on the long term some deterioration of the development of differentiation and so on. So it adds up basically, even If Lifeact on the short term and our experiments might be okay, having them longer experiments might lead to problems.
Vivien
Oh, by the way, short term and long term, so maybe an hour versus?
Roland Wedlich-Söldner
Yeah, so I think these organoid experiments very often they go for weeks, but they wouldn't continuously image them. So that would be enough that every three days you have a look and basically look at some characteristics. And actin is a very nice marker, not just for actin, but for cell shape, for lots of organelles. So you see very often actin associated with endosomes, with ER with Golgi, you can see basically everything in the cell with this single marker. And sometimes just having as a negative marker in the cytosol would also help.
And the main advantage that they see for simply as a marker for for experiment, like we do every day on the short term, minutes or hour timescale, I didn't see that much.
So I think that simply by tuning the concentration of Lifeact , you can get rid of nearly all the effects. So we tried to replicate some of the defects that people have published. And we still don't see them simply. So we can go over two orders of magnitude of Lifeact expression and have no, not even the slightest measurable defects on cell morphology or cell behavior on something.
Vivien
That's kind of weird.
Roland Wedlich-Söldner
I think it's just sometimes to become more sensitive. So you might need to put them into an environment where they have a more elongated cell shape tend to become more sensitive, you need more force exerted by myosin where usual conditions it's not so critical. So I don't find that too surprising. But it generally means that Lifeact is not at a central problem, it can be tuned by conventional means of expression level, or maybe the different fluorophores that you add might also make a difference. So, and I didn't see a pressing issue there to be solved. That would make it like a revolution now with the LILAC. So the LILAC really gives you this one option that was completely new, for a particular type of experiments, niche experiments, I would call them but still important ones, where you would have no option at the moment to have like a completely non detrimental marker.
Vivien
So those are the just for babies, it's the dark phase, right,
Roland Wedlich-Söldner
The dark phase would be completely in non functional and non non disturbing. Convincing in the paper, and also that basically, they show if it's off, basically, they find no phenotype of this doesn't mean that it's always true. So that will again be have to be validated by every experimentalist for the system.
Michael Sixt
You potentially use it to bring something acutely to actin right, to bring something else in. If you want to sequester other molecules or acutely localize other molecules to the cytoskeleton. So that that could of course be Yeah, there might be questions where we could use option. Yeah.
Roland Wedlich-Söldner
So then I think it's basically gives gives a new entry point, but basically would be a future paper showing how that could be applied. Similar bifactor, we've tried to use it as like a simple anchor to bring things to actin, but it's non trivial to use this in a reproducible manner. So you could basically develop drugs on that basis, just like lifeact could protect from certain toxins, you can also basically use Lifeact to locally or temporarily activate certain certain compounds.
Vivien
Perhaps just as one example it could be used to selectively affect muscle.
Roland Wedlich-Söldner [31.20]
And yes, you could basically then affect something selectively for muscle. If you find like, a drug or treatment that would only be active, let's say, if you have a lot of calcium that constantly is activated, that would perfectly work.
Michael Sixt
No, sub cellular targeting of effectors is definitely something that could be interesting.
Vivien
As an aside from the discussion about actin I asked about a recent paper from the Sixt lab which was about the forces in a swelling lymph node. For example due to infection a lymph node can swell to ten times its size in just a few days. Michael Sixt and his colleagues looked at the mechanosensing response involved here looking at two transcription factors that shuttle between cytosol and cell nucleus in response to tension on the cytoskeleton.
Michael Sixt [32.10]
Actually, a lot of people do that. So we use it as a basically as a reporter for is there tension on the cells or not, so are the cells under tension. Because when the lymph node swells then the skeleton of the lymph node, basically, the fibroblasts there, they get extended, because the lymph node swells very quickly, and then there's tension on these internal structural cells, and they get under tension. And so in effect, this transcription factor shuffles, but that has been shown by other people that it does the shuffling. And in this case, we just did stainings of it. And so this is, this is actually, that's not too tricky to do. But it's a, it's a way to read out, indirect read out for intracellular forces.
And this is in general, something where we are still lacking direct reporters to measure intracellular forces. And also actually extracellular forces. So there are a few people who develop tension sensors and so on. But I think this would be super interesting. If we could make Lifeact that reports the tension on the cytoskeleton, a variant of that, so that you really know what kind of forces is the cell producing and experiencing.
Vivien
In this case the reporters, the labels, were proxies, indirect readouts.
Michael Sixt [33.40]
The cell is under tension, and then tons of things happen. And in the end, this transcription factor goes to the nucleus, and we use it as a reporter. But that's not a very satisfying thing. In the end, it would be much more satisfying to directly measure, what is the force on the cell? And how much as you ask it the beginning, right? How much forces does the cell actually produce? And under which conditions and how does this relate and to the deformation of the environment? Where we again, don't know exactly what are the mechanical properties? So finding reporters for that will be very, really interesting.
Vivien
Since we were on the subject of wishlists I thought I should ask what else is on their wishlist for the future. Also as a way to hear what people might consider working on , including in their labs but also beyond their labs. First Roland Wedlich-Söldner:
Roland Weidlich-Söldner [33.35]
In the very narrow field of what wish list we would have for the actin imaging analysis. They are very clear things that are still missing that Lifeact cannot cover and we were touching on that before. Because Lifeact is so weakly binding, it basically goes on and off on a millisecond timescale. So basically, while you can see actin labeled by Lifeact, each individual Lifeact doesn't stay say for more than a few milliseconds track that act in Dynamics directly using life act, because if you look at a growth of an actin filament, the Lifeact will simply follow that, but you cannot really look at the turnover.
So there is still no marker whatsoever that does not directly interfere with the dynamics. So actin itself can be labeled, but it's not functional in full sense. So there's no marker to look at it, actin turnover in a cell or in a living environment, and also correlated or linked to that, to measure what is the actual pool of monomers that is available to the cell at any given time and position. Because we know there's a lot of actin around, but most of that is not available for making anything useful out of it.
They're basically just sequestered by other proteins and sticking in the cytosol cannot be used. So there's a usable pool. And nobody knows how big that actually is. There's a lot of discussion, a lot of attempts to measure it, very complicated, means take years of work, and the end they're not satisfying. So there's a clear big gap still in the field that Lifeact cannot address. And yeah, actually, with ideas, how to maybe approach it, that there's ways and maybe label actin directly that is not interfering, and we try that there. Again, yeast is very helpful. Because you can basically replace endogenous proteins more simply tha n a mammalian system, but that's unsolved. And yeah, not sure how that or when that will happen. But it's a very narrow wish list, obviously.
Vivien
And he also pointed out another probe, one from the lab of Uri Manor at the Salk Institute
Roland Wedlich-Söldner [36.40]
This other thing that was in Nature Methods not too long ago from Uri Manor was on basically a Lifeact on nanobody not Lifeact itself, but an actin binding probe attached to mitochondria to organelles, to see subsets of actin in there.
But they always have this issue that you might concentrate it actin by simply binding to it and the same thing with a nuclear localization effect, just by putting it into the nucleus, you suddenly have like 10 times smaller volume, the meaning 10 times higher concentration, which tends directly to these phenotypes of Lifeact stabilizing actin by blocking cofilin for example, and then you get actin in the nucleus , zs, but only because the life is that so there Let's see what you didn't want to generate in first place. So there was a lot of controversy in the first papers that use that until they could develop appropriate controls that it really happens.
Vivien
And here is Michael Sixt and his wishlist for the future.
Michael Sixt
When you when you think of actin, it's it's everywhere in the cell, right. So it's the most abundant protein in eukaryotic cells, right. And so the cell is full of it. And I think it would be really interesting to get better tools to look at sub-cellular subsets of actin, right. Just selectively look at actin on the mitochondria, on the different organelles, and so on. And people have done that for plasma membrane, actually binding an actin marker to the plasma membrane to selectively look at what is actually directly, below the membrane. And, and I think this in the nucleus, that's another thing. There's actin in the nucleus. But it's so little, that you never see it. Because you have to crank up the volume, so much that everything, everything else, shines . So people did put Lifeact in the nucleus by putting a nuclear localization site on, and then you suddenly see the actin the nucleus.
Vivien
That was Conversations with scientists.
Today's guests were Michael Sixt at the Institute of Science and Technology Austria and Roland Wedlich-Söldner from the University of Münster.
The music used in the project is Funky Energetic Intro by Winnie the Mook licensed from film music.io and Rice Crackers from Aves, licensed from Artlist.io.
And I just wanted to says because there's confusion about these things sometimes.
The University of Münster or the Institute of Science and Technology Austria didn't pay for this podcast. And nobody paid to be in this podcast. This is independent journalism that I produce in my living-room. I'm Vivien Marx, thanks for listening.
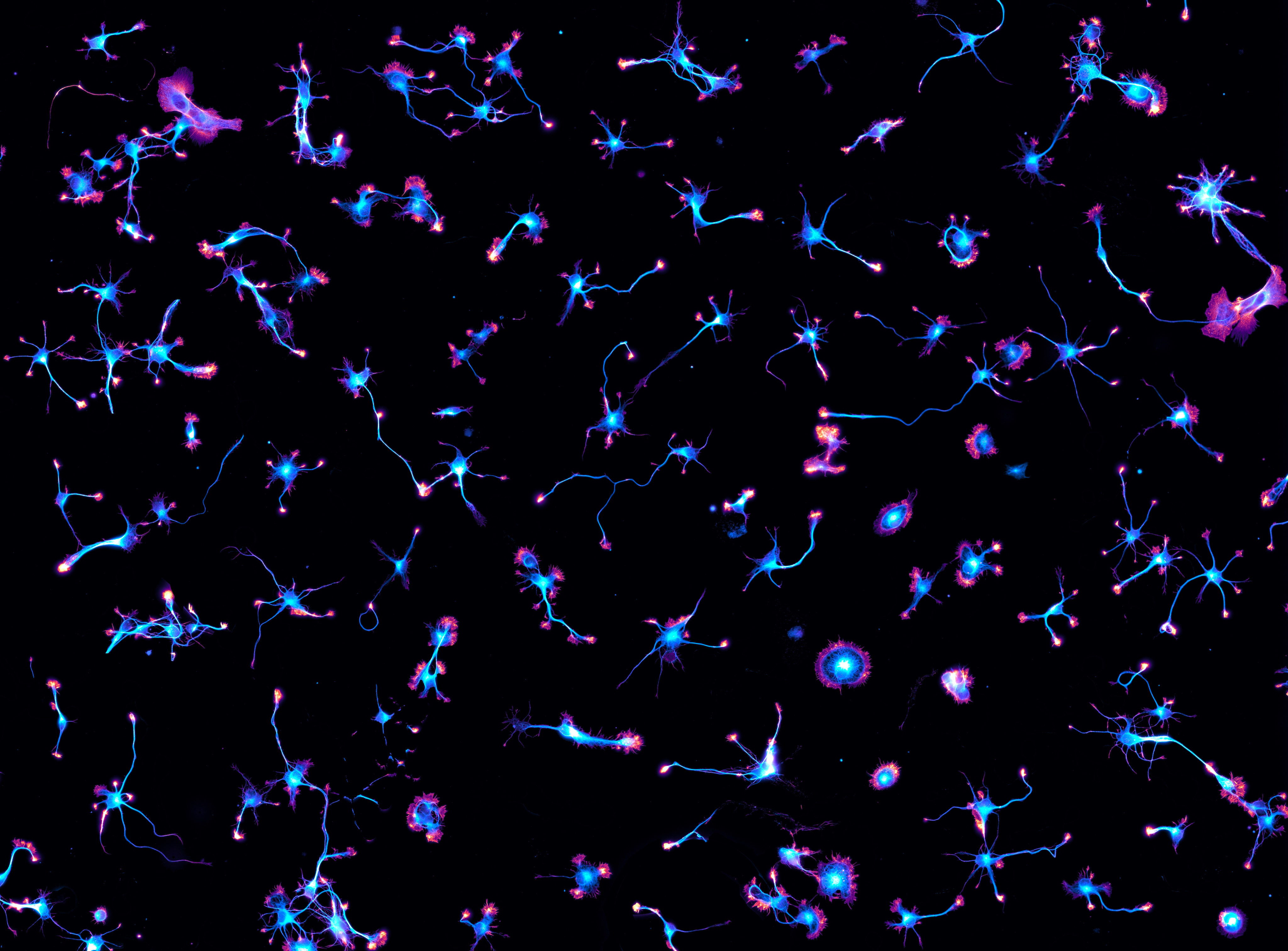
(Credit: Christophe Leterrier, Centre National de la Recherche Scientifique (CNRS) the Institute of NeuroPhysiopathology, which is affiliated with CNRS and Aix Marseille University)
Follow the Topic
-
Nature Methods

This journal is a forum for the publication of novel methods and significant improvements to tried-and-tested basic research techniques in the life sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Methods development in Cryo-ET and in situ structural determination
Publishing Model: Hybrid
Deadline: Jul 28, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in