DARPP‑32 Beyond the Brain: A Potential Regulator of Macrophage M2 Polarization Through STAT6 Signalling
Published in Cancer and Anatomy & Physiology
Sangita Chowdhury, Dr. Arun Kumar Trivedi
Published in Oncogene (10.1038/s41388-025-03610-x)
Date: 24 October 2025
A suitable ecological niche for the onset and spreads of cancer is provided by the surrounding tumor microenvironment (TME). Major components of the TME are immune cells and mediators. Tumor-associated macrophages (TAMs) have been used as a model to illustrate the relationship between inflammation and cancer progression. TAMs predominantly exhibit an M2-like phenotype within the tumor microenvironment (TME), where they contribute to immunosuppression, tissue remodelling, and tumor progression. Thus, identifying novel regulators of macrophage M2 polarization is essential for understanding immune modulation in diseases including cancers.
DARPP-32 (Dopamine- and cAMP-regulated phosphoprotein of 32 kDa) is predominantly expressed in neurons of the striatum and functions as a molecular switch that integrates neurotransmitter signals by modulating the activity of protein kinases and phosphatases, depending on its phosphorylation state. When phosphorylated at threonine 34 (Thr34) by protein kinase A (PKA) or protein kinase G (PKG), DARPP-32 becomes a potent inhibitor of protein phosphatase-1 (PP1). This inhibition enhances downstream phosphorylation events, thereby amplifying dopamine D1 receptor signalling. In contrast, phosphorylation at threonine 75 (Thr75) by cyclin-dependent kinase 5 (Cdk5) converts DARPP-32 into an inhibitor of PKA itself, attenuating cAMP-mediated signalling.
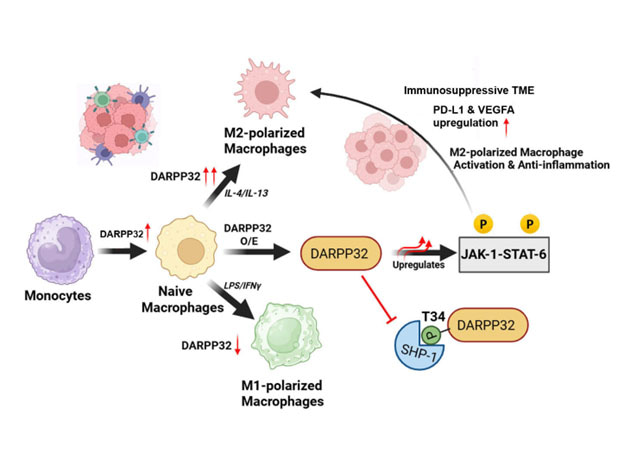
Here we demonstrate that DARPP-32, a protein phosphatase inhibitor, is significantly upregulated in FBXW7 knockout THP-1 cells that spontaneously adopt the M2 like phenotype and characteristics. DARPP-32 expression increases markedly in macrophages exposed to diverse M2-inducing stimuli (IL-4, IL-13, IL-10 and TGF-β), while it decreases following treatment with M1 inducers such as lipopolysaccharide (LPS) and IFN-γ. In contrast, DARPP-32 depletion impairs IL-4–mediated M2 differentiation of macrophages. Additionally, DARPP-32 overexpression suppresses the classical M1 activation program in response to LPS/ IFN-γ, shifting macrophages toward an anti-inflammatory M2-like phenotype.
Based on DARPP-32 varying expression, we proposed that DARPP-32 might also serve as a marker of M2-polarized state along with other well-established M2 markers. Mechanistically, Thr34-phosphorylated DARPP-32 enhances JAK1-STAT6 signalling by directly binding and inhibiting the protein tyrosine phosphatase SHP-1, a critical regulator of immune signalling and macrophage activation.
Corroborating its role in tumor-associated macrophages, DARPP-32 is markedly elevated in tumor-infiltrating macrophages within primary triple-negative breast cancer (TNBC) patient samples and in macrophages co-cultured with tumor cells. DARPP-32 promotes immune-suppressive functions in the tumor microenvironment by increasing PD-L1 and VEGFA levels, suggesting its potential as a therapeutic target for reprogramming TAMs and enhancing anti-tumor immunity. To our knowledge, this is the first comprehensive study identifying DARPP-32 as a key driver and regulator of macrophage polarization toward the immunosuppressive M2 phenotype.
Translational Significance
M2/TAM-associated genes/proteins can serve as biomarkers for tumor aggressiveness, prognosis, or response to immunotherapy (e.g., high TAM infiltration often correlates with poor outcomes). Potent inhibitory effect of DARPP-32 on pro-inflammatory M1 macrophage polarization represents a potential therapeutic target in cancer for reactivating macrophage-mediated immune responses. Inhibition of DARPP-32 may promote M1 polarization and enhance anti-tumor immunity.
Additionally, pharmacological activation of protein phosphatases, counteracting the effects of DARPP-32 could serve as a long-term strategy to suppress M2-like tumor-associated macrophage (TAM) polarization and mitigate immunosuppressive tumor microenvironments (TME). DARPP-32 drives macrophage M2 polarization to promote an immunosuppressive tumor microenvironment and impairs T-cell activation.
According to the literature, macrophages comprise nearly 50% of the cellular population in the tumor microenvironment and are the predominant contributors of PD-L1 expression, which inhibits T-cell activity. Thus, identification of upstream regulatory proteins that control PD-L1 (Programmed Death-Ligand 1) expression is of translational importance in cancer immunotherapy.
As we showed DARPP-32 also regulates PD-L1 expression in macrophages treated with tumor-conditioned media (TCM), our finding holds significant therapeutic potential. Dissection of the molecular network governing DARPP-32 mediated PD-L1 expression may further provide insights for better optimization of current immunotherapeutic strategies and developing next-generation immune modulators.
As, we also observed that DARPP-32 inhibits T-cell activation and proliferation, mechanisms underlying DARPP-32's role in mediating tumor immune evasion by directly inhibiting T cell-mediated tumor killing is solely-mediated through PD-L1 axis or involves additional pathways warrants further exploration.
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in