Deciphering the effects of COVID-19 on the Brain
Published in Neuroscience

The worlwide pandemic caused by the novel coronavirus, SARS-CoV-2, has severely impacted our everyday life, and the consequences of the disease caused by the virus (known as COVID-19) are still burdening the life of numerous persons, in some cases even after infection has waned.
Although manifesting primarily as a respiratory disease, neurological symptoms are common in COVID-19, either presenting as new manifestations or by worsening pre-existing conditions. Mild symptoms, commonly encountered by a wide range of patients, include loss of taste and smell (ageusia and anosmia), dizzyness, fatigue and sleep disorders, among others. More rarely, severe neurological conditions may arise, such as cognitive impairment, altered mental status, brain inflammation (known as encephalitis), and stroke. Between 10 to 30% of COVID-19 patients experience long-term consequences of infection, with persisting symptoms even after the virus becomes undetectable (i.e. conventional testing becomes negative). This condition is referred to as “Long COVID”, and represents one of the most burdening consequences of the SARS-CoV-2 pandemic, along with the psychological and economical issues connected with the pandemic.
These observations raise the question of whether the novel coronavirus possesses neurotropic properties; that is, whether the virus is able to infect the cells of the nervous system or not. In fact, it is yet unclear whether neurological symptoms are caused by the direct infection of the brain, or if they are more likely related to the effects of infection on the rest of the body, particularly the respiratory system, and the consequent immunitary reaction. While this can be investigated in various ways, the most accurate approach to decypher the pathology underlying neurological symptoms in COVID-19 is known as neuropathology, the study of diseases of the nervous system that allows for the examination of the brain of deceased persons.
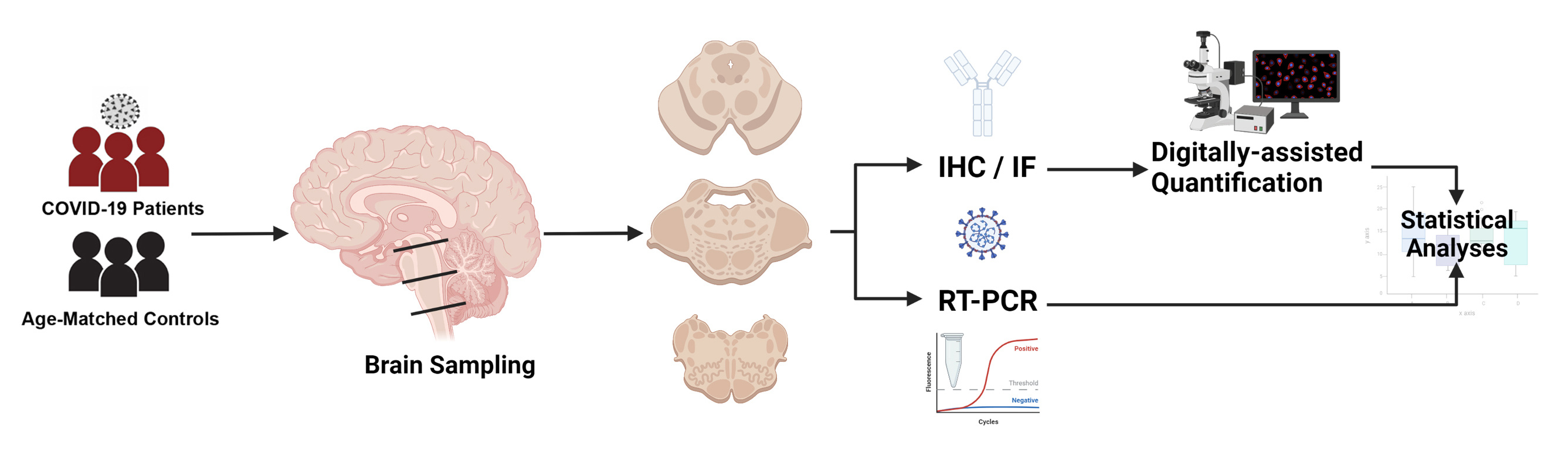
In this study, published in Nature Partner Journal (NPJ) Parkinson's Disease, we have examined the effects of SARS-CoV-2 and COVID-19 on the brain of patients who died during the first wave of the pandemic in Italy. Our country, and the Veneto region in particular, were among the first regions worldwide to experience the effects of the SARS-CoV-2 pandemic already back in 2020, and the rising reports of neurological manifestations in COVID-19, togheter with our interest in the nervous system anatomy and pathology, have led to the definition of the current study. Starting with a small set of 4 cases, among the first authorized COVID-19 autopsies in our region, we quickly expanded our study to 24 decedents. Prior to infection, our patients were affected by significant medical comorbidities, i.e. other occurring pathologies such as increased blood pressure, diabetes, renal insufficiency, etc.; hence, it was necessary to discern between pathological changes in the brain determined by age and prior pathologies, and the effects of COVID-19. For this purpose, we decided to compare our COVID-19 cohort with age- and sex- matched subjects who had similar medical comorbidities, and who mainly died due to pneumonia, an inflammatory condition of the lungs caused by viruses or bacteria (in this instance, not SARS-CoV-2). Pneumonia and respiratory infections are known to cause inflammation that can also affect the brain; hence, comparing brain findings in COVID-19 to those occurring in pneumonia can provide a more realistic measure of the severity of the disease, allowing us to determine how COVID-19 differs from other respiratory infections. In our cohort, we extensively sampled the brain, and focused on the brainstem. This is an anatomical region involved in the regulation with numerous body functions, such as control of breathing and heart rate modulation. The brainstem is often affected in viral infections and is known to be invaded by other coronaviruses, such as the middle easter coronavirus and SARS-CoV, thus representing an region of particular interest. We studied COVID-19 neuropathology through immunohistochemistry, a technique that allows for the detection of specific tissue components, such as inflammatory cells or even the structural proteins of the coronavirus, while we also employed molecular techniques to detect viral genomic sequences.
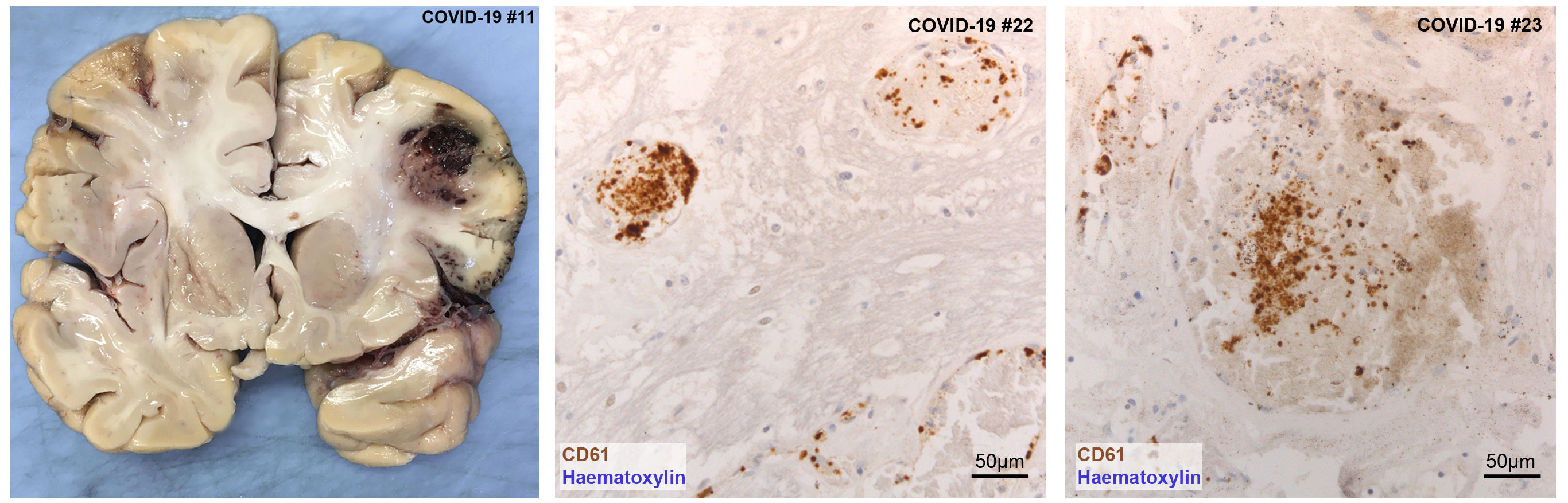
 We detected a wide spectrum of pathological alterations in the brains of both COVID-19 and control subjects, although numerous findings were not specific to COVID-19 and better explained by age and prior medical conditions. Both COVID-19 and pneumonia patients presented brain suffering and damage due to reduced oxygen (hypoxia) and blood (ischemia) supply, even though damage was more severe in COVID-19. Small vessel thromboses, which are blood clots occluding a blood vessel, were found only in COVID-19 patients, and often affected multiple organs (such as the lungs and liver).
We detected a wide spectrum of pathological alterations in the brains of both COVID-19 and control subjects, although numerous findings were not specific to COVID-19 and better explained by age and prior medical conditions. Both COVID-19 and pneumonia patients presented brain suffering and damage due to reduced oxygen (hypoxia) and blood (ischemia) supply, even though damage was more severe in COVID-19. Small vessel thromboses, which are blood clots occluding a blood vessel, were found only in COVID-19 patients, and often affected multiple organs (such as the lungs and liver).
SARS-CoV-2 viral proteins and viral RNA were detected in the brainstem of 5 COVID-19 subjects. Specifically, SARS-CoV-2 viral proteins were found in neurons of specific brainstem nuclei, giving us potential information on the entry routes exploited by the novel coronavirus. The affected regions included the nuclei of the vagus nerve, whose axons innervate the lungs, heart and bowels. Prior studies identified the vagus nerve as a potential route for SARS-CoV-2 brain entry, and the identification of viral proteins in the nuclei of this nerve strongly support this notion. As the vagal nuclei contribute to the regulation of breath rhythm and heart activity, disruption of vagal neurons by SARS-CoV-2 can further worsen the respiratory conditions of COVID-19 patients, which are already compromised by lung damage. We also found viral proteins and RNA in the midbrain, and in particular in a region of the midbrain called substantia nigra. This part of the brain is known to play a critical role in the modulation of motor circuits, and its degeneration is associated to Parkinsonism, one of the clinical manifestations of Parkinson's Disease. Viral infections are associated to and significantly increase the risk of neurodegenerative diseases, in particular Parkinson's Disease. Hence why the detection of coronavirus proteins in these neurons is particularly intriguing, as well as concerning. (more information on the possible link between viral infection, antiviral response, inflammation and neurodegeneration can be found in the discussion section of our manuscript).
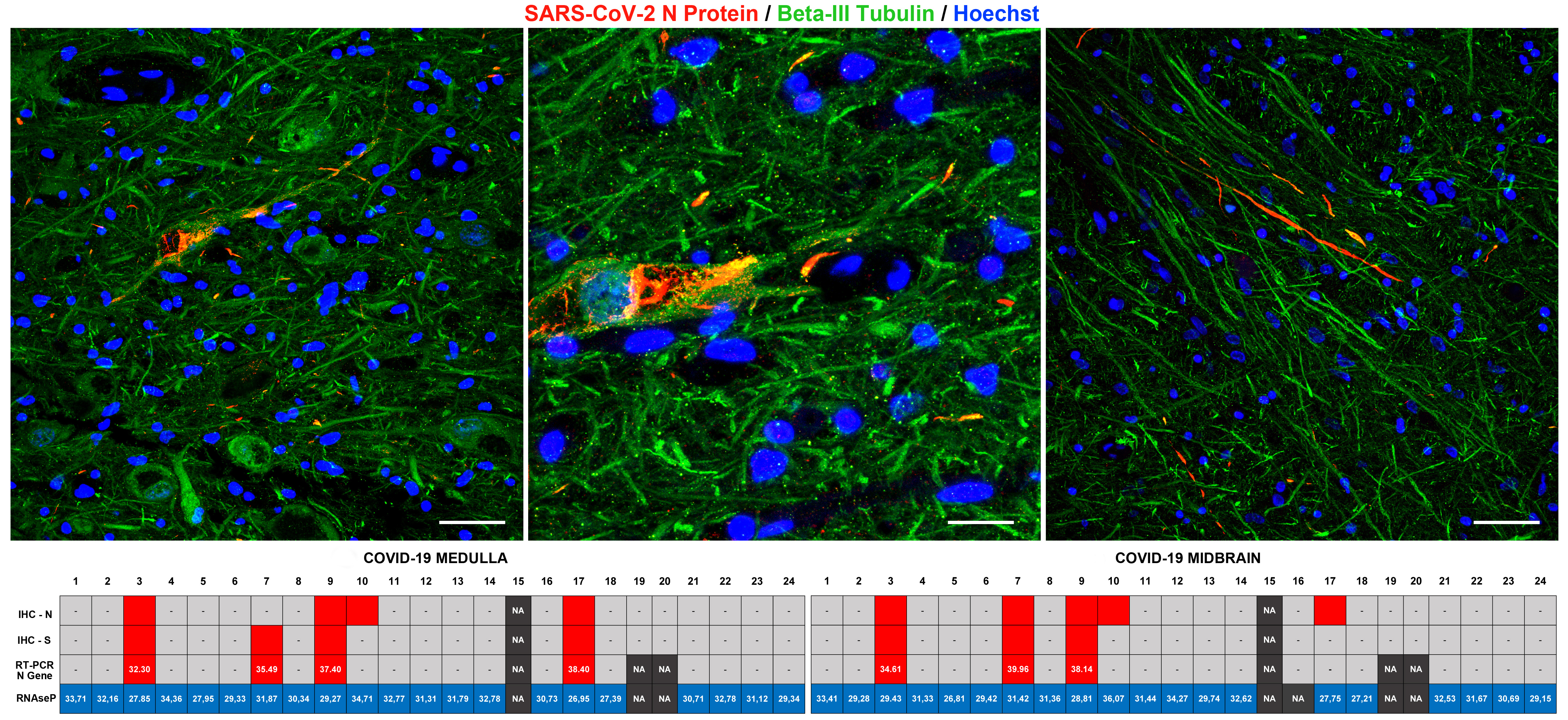
While SARS-CoV-2 viral proteins were present in neurons, we did not detect indicators of direct neuronal damage caused by the virus. Indeed, even if markers of infection were present, these neurons appeared relatively unaffected. This can be explained by the fact that all of our COVID-19 patients died during the acute phases of infection and within a relatively short timeframe from hospitalization (up to 38 days), so we hypothesized that direct damage to neurons induced by the virus did not have time to develop at the time of death. This, however, remains to be further elucidated.
Conversely, prominent inflammatory pathology was found. Resident immune cells of the brain, known as microglia, displayed an activated phenotype with increased phagocytic activity. The phenotype of microglial cells was also different when compared to our control group, indicating prominent activation in COVID-19.
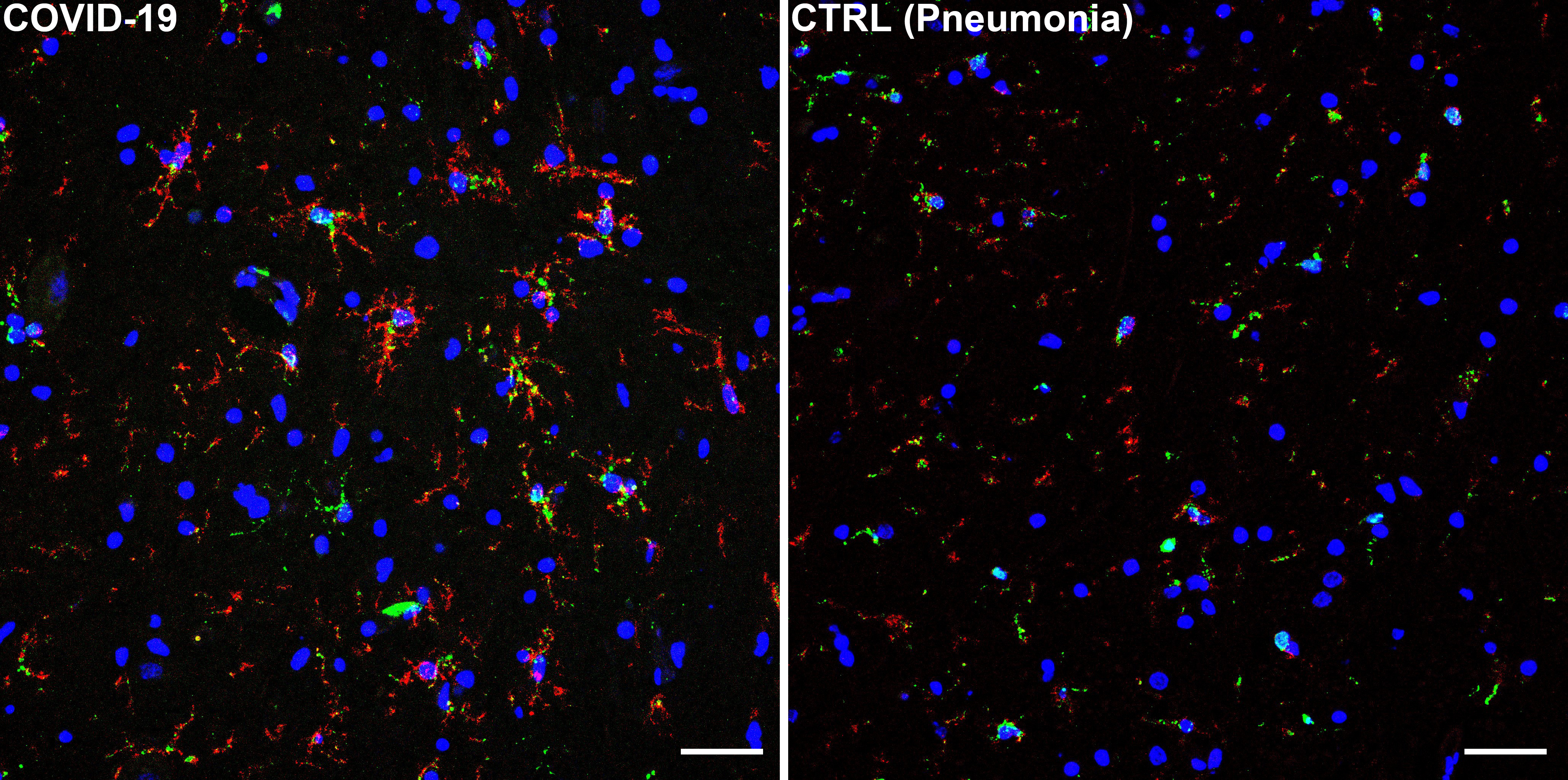
Microglial cells were frequently found in clusters, comprising also other cellular elements, such as neurons and lymphocytes. These clusters, known as microglial nodules, are typical features of viral infections and were detected in numerous brain regions of COVID-19 subjects. Microglial nodules were highly suggestive of neuronophagia, the process that leads to the phagocytosis of damaged or necrotic neurons.
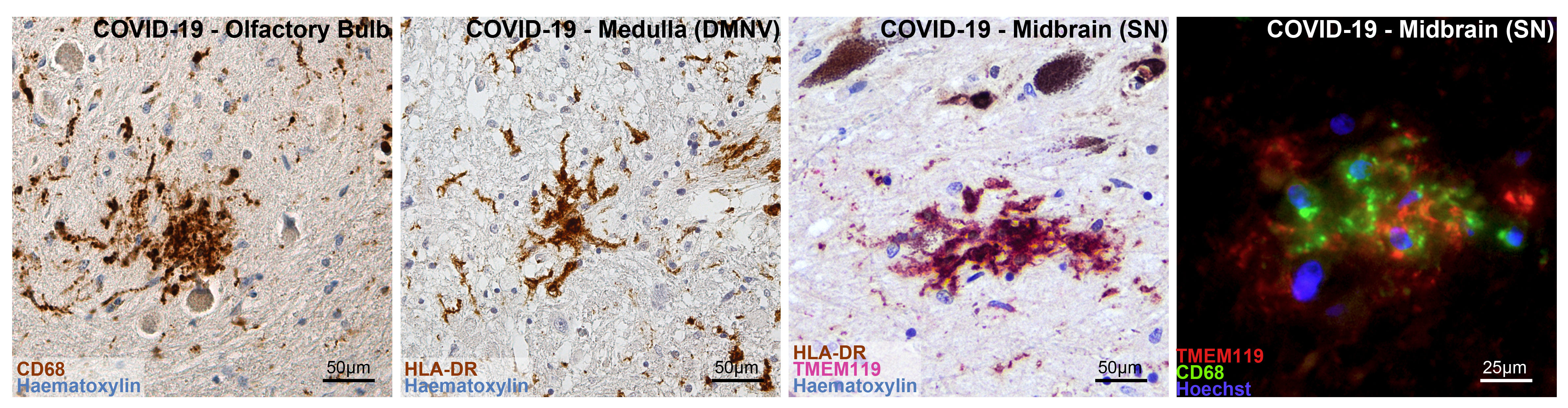
Lastly, we analyzed the spatial distribution of microglial cells at various brainstem levels. We detected prominent differences between COVID-19 subjects and pneumonia controls, and also evidenced an anatomically-defined pattern of microgliosis in the examined sections. Simply put, microglial cells did not distribute evenly throughout the brainstem, but were more densely localized in specific regions, in particular the dorsal medulla, where the nuclei of the vagaus nerve are found, and the ventral midbrain, at the level of the substantia nigra. Intriguingly, these were the same regions in which we also detected viral proteins in a subset of COVID-19 cases. Hence, we hypothesized a possible relationship between microglial densities and the presence of viral proteins. Our results suggest that COVID-19 subjects with viral proteins in brainstem nuclei present higher local microglial densities compared to COVID-19 subjects whitout. On the other hand, we also found an important correlation between microglial densities and hypoxic-ischaemic damage, suggesting a prominent role of this type of brain pathology in mediating inflammation.
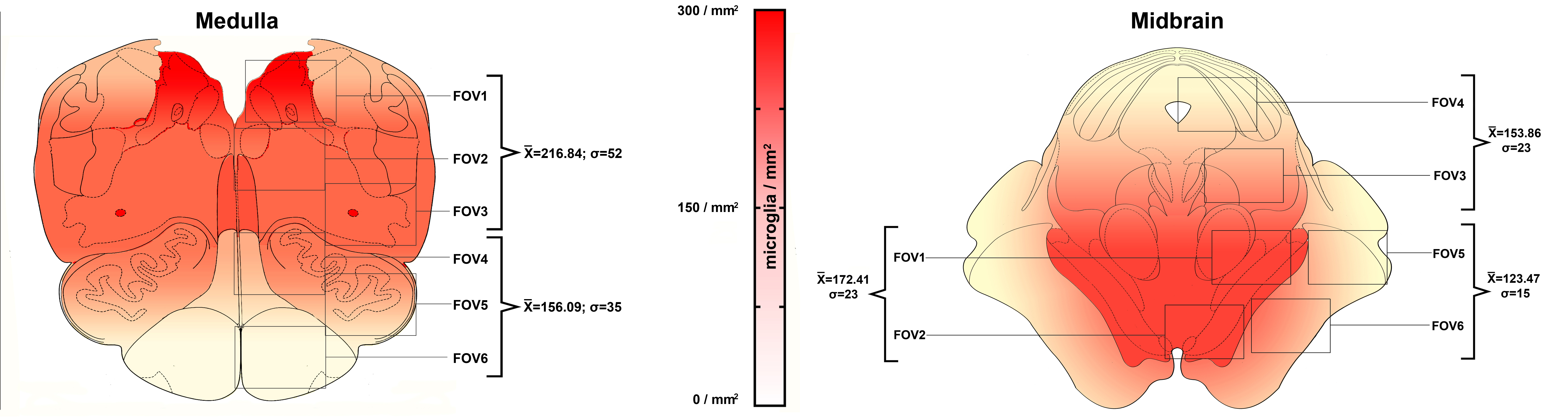
In conclusion, our study identifies SARS-CoV-2 viral proteins in specific brainstem nuclei in a subset of COVID-19 decedents. Microglial cells presented an activated phenotype and expressed pro-inflammatory markers, while quantification of reactive microglia revealed an anatomically segregated pattern of inflammation within affected brainstem regions. Further investigation is required to determine whether or not SARS-CoV-2 neurotropism represents a major component of COVID-19 in the general population, as subjects included in neuropathological studies often present a much more severe course of the disease and major medical comorbidities. Nevertheless, the findings of our study suggest the possibility that, although not frequently, SARS-CoV-2 may gain access to specific regions of the central nervous system. As direct neuropathological alterations determined by SARS-CoV-2 neurotropism may not be detectable in subjects deceased during the acute stages of the disease, future studies are required to determine whether or not SARS-CoV-2 neurotropism is present in chronic COVID-19 patients, or in COVID-19 survivors suffering from the long-term effects of infection.
Follow the Topic
-
npj Parkinson's Disease

This journal publishes original basic science, translational and clinical research related to Parkinson's disease, including anatomy, etiology, genetics, cellular and molecular physiology, neurophysiology, epidemiology and therapeutic development and treatments.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Cognition - preclinical models, and preclinical unmet need
Publishing Model: Open Access
Deadline: Jul 27, 2026
Environmental risk factors for Parkinson’s disease
Publishing Model: Open Access
Deadline: May 13, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in