Declines in alkaline phosphatase and pain during radium-223 treatment may predict longer survival in patients with advanced prostate cancer
Published in Cancer and General & Internal Medicine
Radium-223 for advanced prostate cancer: Why is it important to identify patients who may respond better to treatment?
One of the life-prolonging treatments available for metastatic castration-resistant prostate cancer (mCRPC) is radium‑223, a systemic radiation therapy that targets prostate cancer that has spread to the bones. Bone metastases occur in the majority of patients with mCRPC and can lead to skeletal complications and pain, which can reduce quality of life and survival. Being similar to calcium, radium-223 (administered via injection into a vein) is taken up in regions of increased new bone formation (a common feature of prostate cancer bone metastases), where it produces radiation that can kill nearby cells by causing DNA damage (Figure 1).
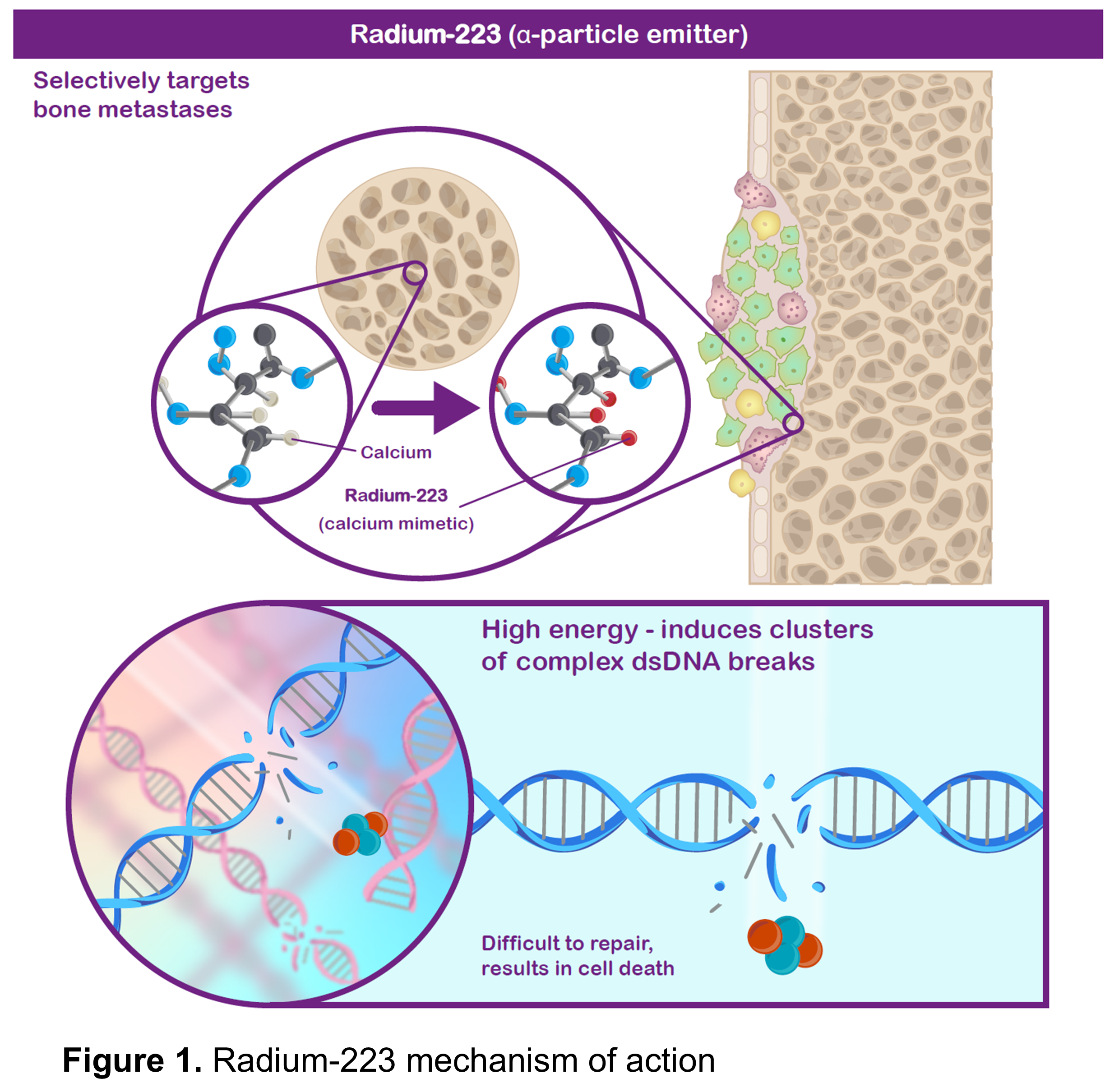
To decide whether a patient should complete a course of radium-223, it is important to assess how their cancer responded to the treatment. However, there are no validated markers to determine outcomes in patients treated with radium-223. This research looks at two possible factors for assessing responses to radium-223: pain and a protein called alkaline phosphatase (ALP).
- Pain is common in patients with advanced prostate cancer and can indicate worsening of the disease.
- ALP levels are sometimes monitored by doctors, as high levels may suggest that the cancer has spread to the bone.
If pain or ALP levels go down during treatment, it may suggest that radium-223 is killing the cancer cells in the bone. Here we looked at whether it is possible to predict survival in patients receiving radium-223 based on early changes in pain and ALP using data collected from REASSURE (an ongoing, prospective, worldwide, long-term study in patients with mCRPC treated with radium-223 in routine clinical practice).
How was the study conducted?
This was a post hoc analysis of REASSURE in which we investigated the associations of ALP decline and/or pain response during radium-223 treatment with survival to explore their potential as efficacy markers. Both ALP levels and pain were measured at baseline. ALP levels were measured again after 12 weeks of treatment and pain was measured throughout radium-223 treatment, to see if there were any changes. Patients were asked to rate their worst pain in the last 24 hours using the Brief Pain Inventory-Short Form (BPI-SF) questionnaire. Pain was rated on a scale of 0 (no pain) to 10 (pain as bad as you can imagine). Patients who had a score of 2 or more at baseline were considered to be in pain. If a patient had a decrease in score of 2 or more during treatment, they were considered to have had a reduction in pain (i.e., a pain response).
What did the study find?
A total of 779 patients had ALP measurements at both baseline and Week 12. Of these, 624 patients (80%) had a decline in ALP at Week 12 and 155 patients (20%) had no decline. Patients who had a decline in ALP were more likely to complete a full course of six radium-223 injections than those with no ALP decline (74% vs. 59%, respectively). We found that:
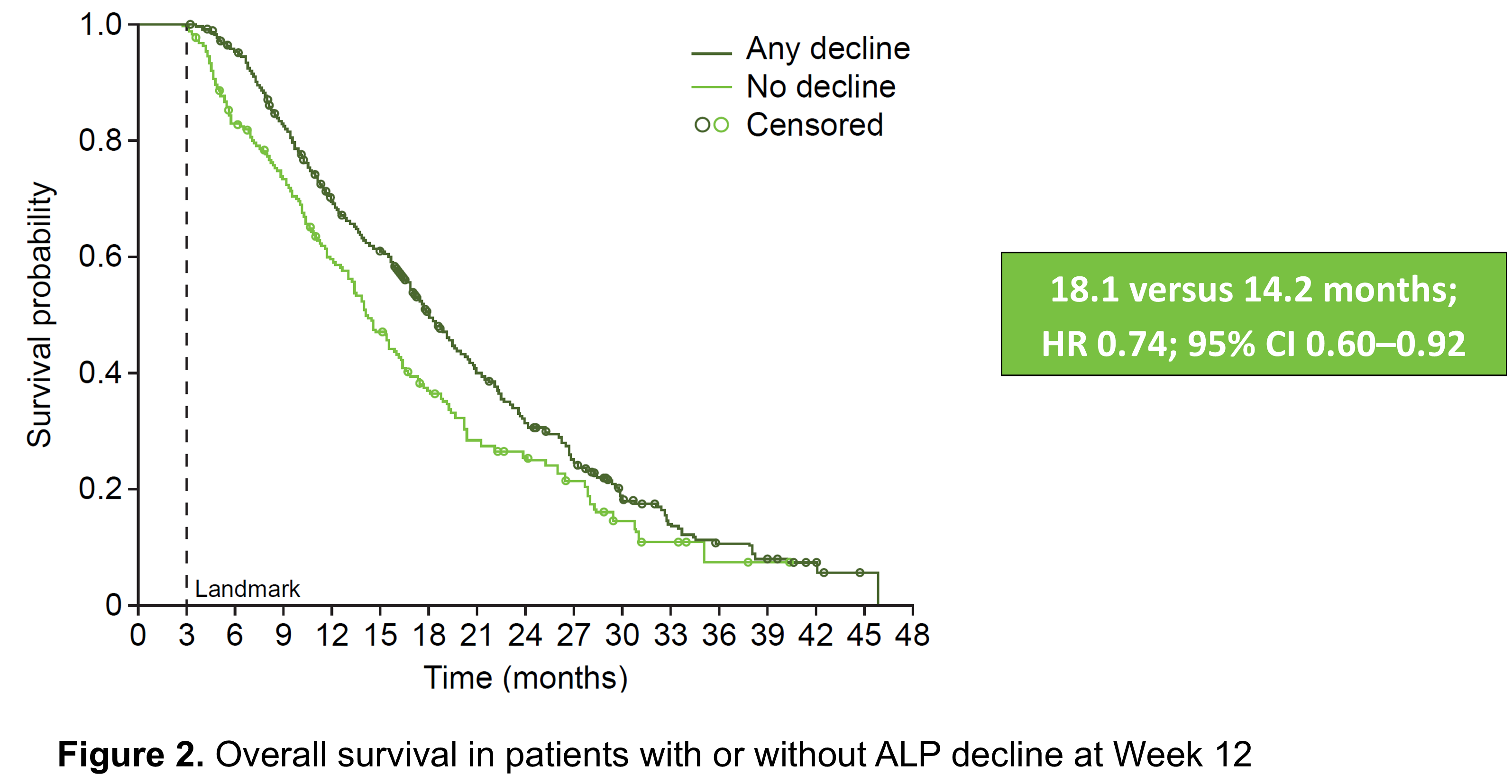
- Patients who had a drop in ALP levels during the first 12 weeks of radium-223 treatment had a longer median overall survival than those who did not (18 months vs. 14 months, respectively; Figure 2).
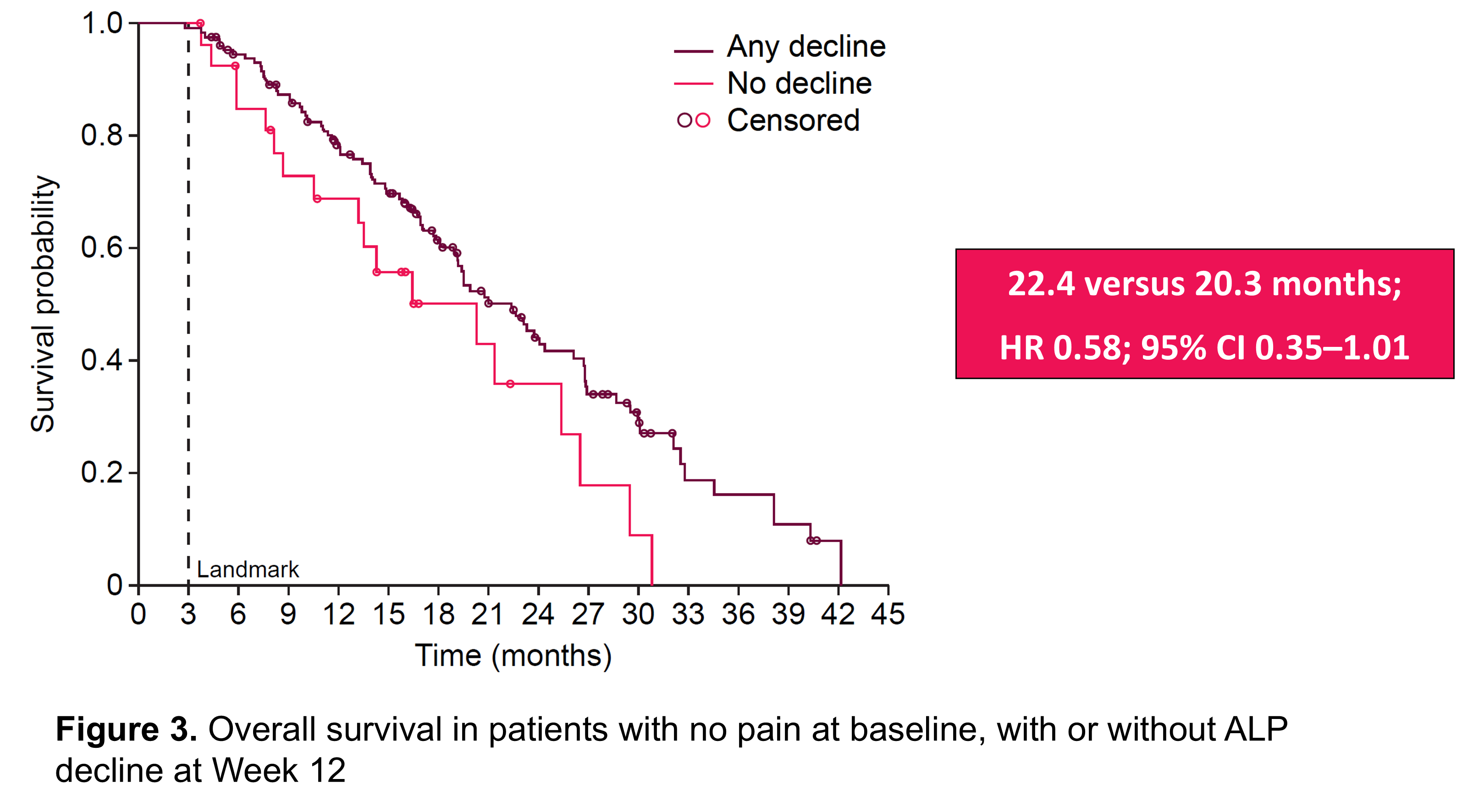
- For patients with no pain at baseline, median overall survival was similar in those with or without a drop in ALP during the first 12 weeks of radium-223 treatment (22 months vs. 20 months, respectively; Figure 3).
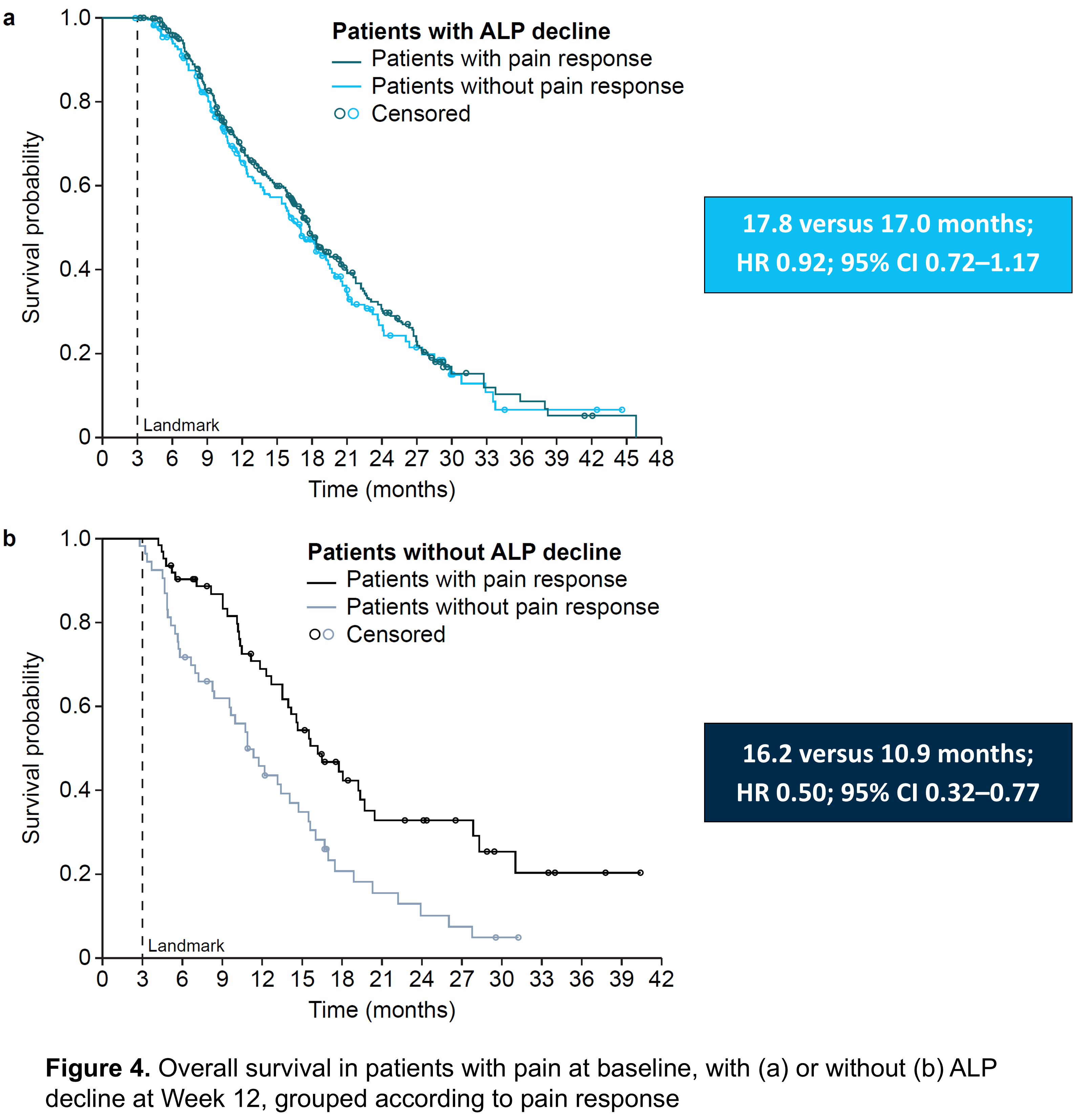
- Patients who had pain at baseline and a drop in ALP during the first 12 weeks of radium-223 treatment had similar median overall survival regardless of whether they had a reduction in pain during treatment or not (18 months vs. 17 months, respectively; Figure 4a).
- Patients who had pain at baseline but did not have a reduction in ALP during the first 12 weeks of radium-223 treatment had a longer median overall survival if their pain reduced during treatment than if it did not (16 months vs. 11 months, respectively; Figure 4b).
What do these results mean?
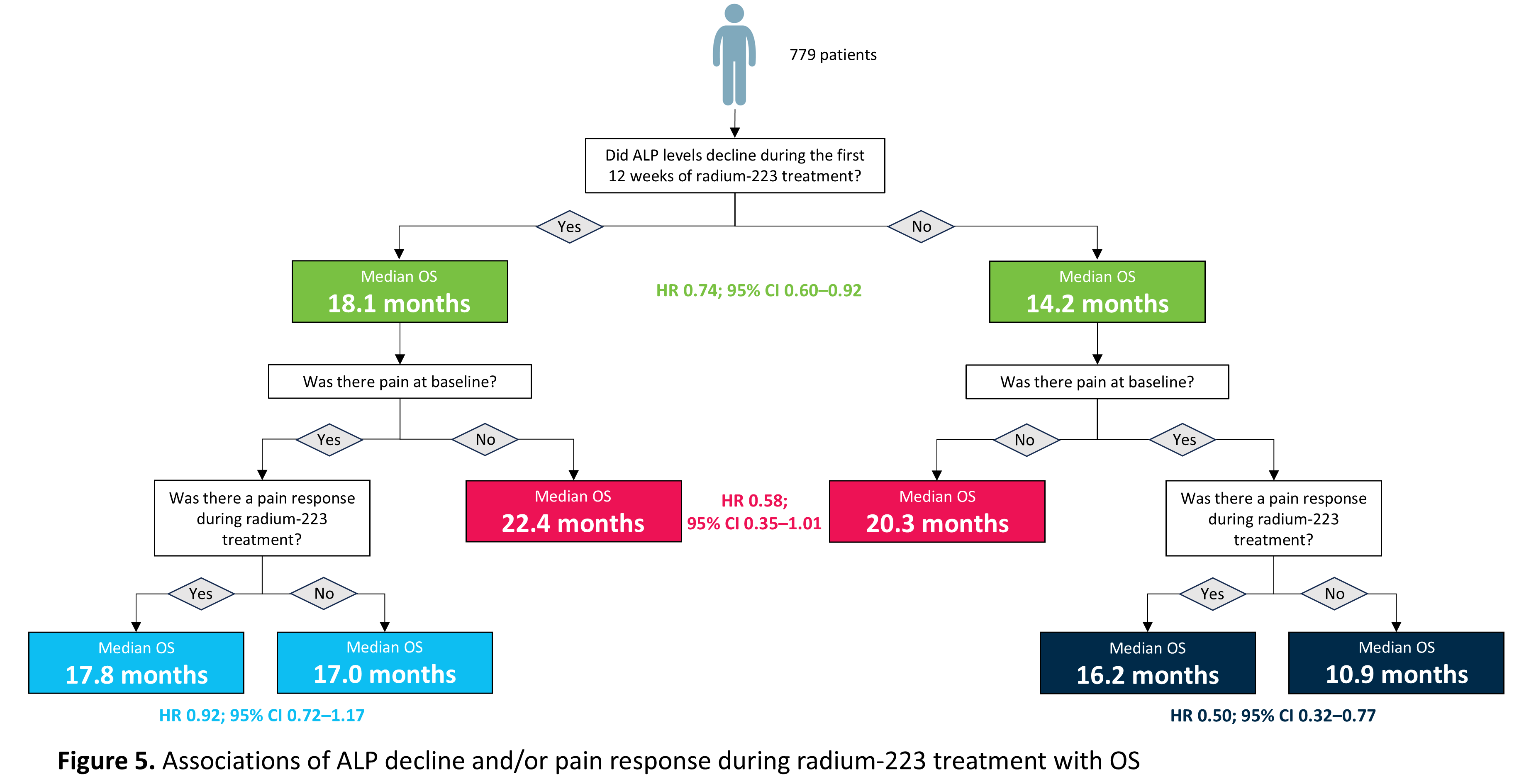
As summarised in Figure 5, declines in ALP or pain may help to predict how long a patient treated with radium-223 may survive, with the best marker combination for longer overall survival being no pain at baseline and an ALP decline during treatment. As survival was shortest in those with no ALP decline, pain at baseline and no pain response during treatment, further clinical evaluation in these patients is warranted. Thus, ALP and pain may serve as predictive markers of survival in patients with mCRPC with bone metastases treated with radium-223 and may help inform clinical decisions.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in