"Deconstruction" of cardiac development and the evolution of tunicate life styles.
Published in Ecology & Evolution

Behind the paper: “Cardiopharyngeal deconstruction and ancestral tunicate sessility”. Alfonso Ferrández-Roldán, Marc Fabregà-Torrus*, Gaspar Sánchez-Serna*, Enya Duran-Bello, Martí Joaquín-Lluís, Paula Bujosa, Marcos Plana-Carmona, Jordi Garcia-Fernàndez, Ricard Albalat, Cristian Cañestro#. Nature (2021) Nov;599(7885):431-435. DOI: 10.1038/s41586-021-04041-w Epub 2021 Nov 17. PMID: 34789899 FREE-ACCESS: https://t.co/4rJA2MURKX?amp=1
Our article by Alfonso Ferrández et al. in Nature is the result of his Ph.D. thesis project together with the contribution of other team members @evodevogenomeUB during many years of research @GeneticsUB @IRBioUB in the University of Barcelona investigating the origin and evolution of our own phylum, the chordates, paying special attention to the impact of ‘gene loss’ as an evolutionary force1.
I will explain the story behind the paper, not only focusing on the scientific impact of the results, but telling other anecdotal aspects that have also been essential for the journey that has made this work possible.
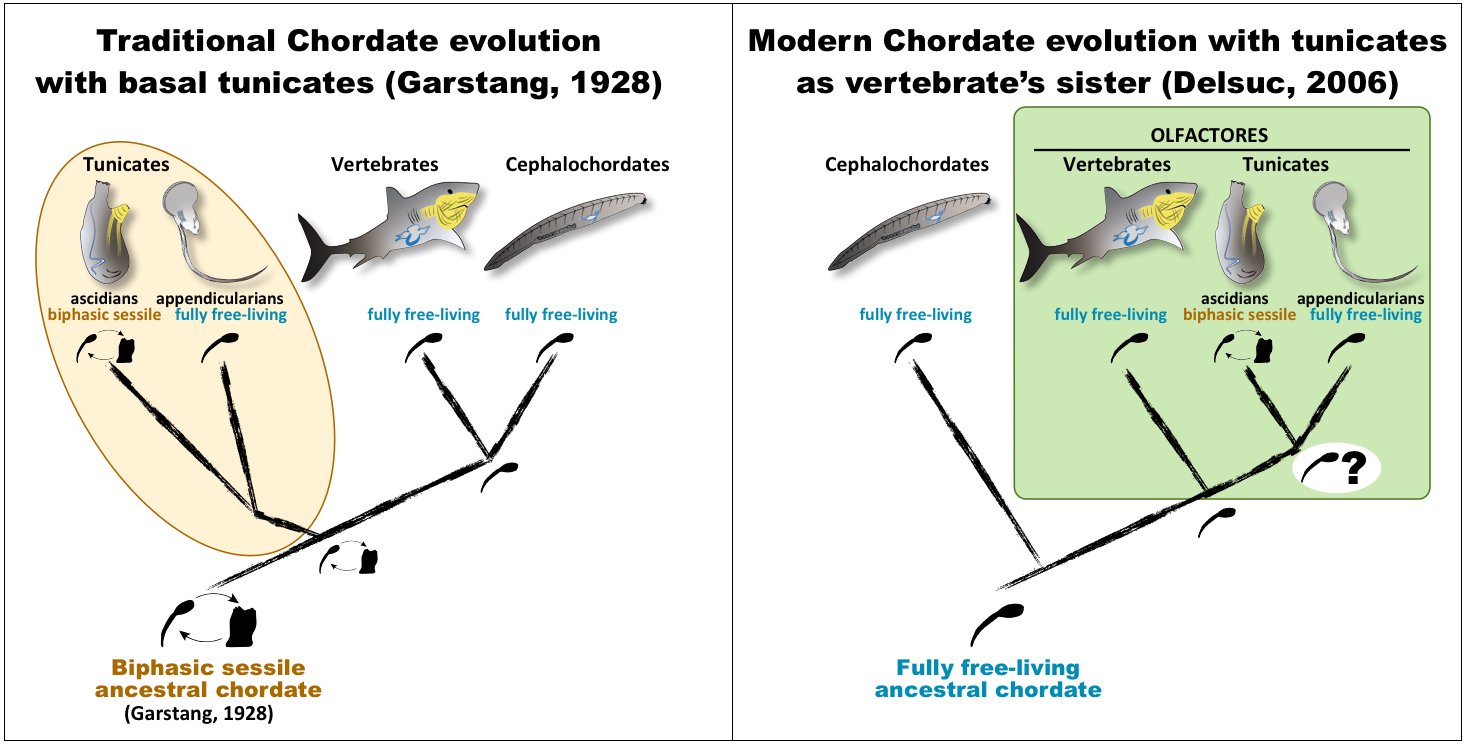
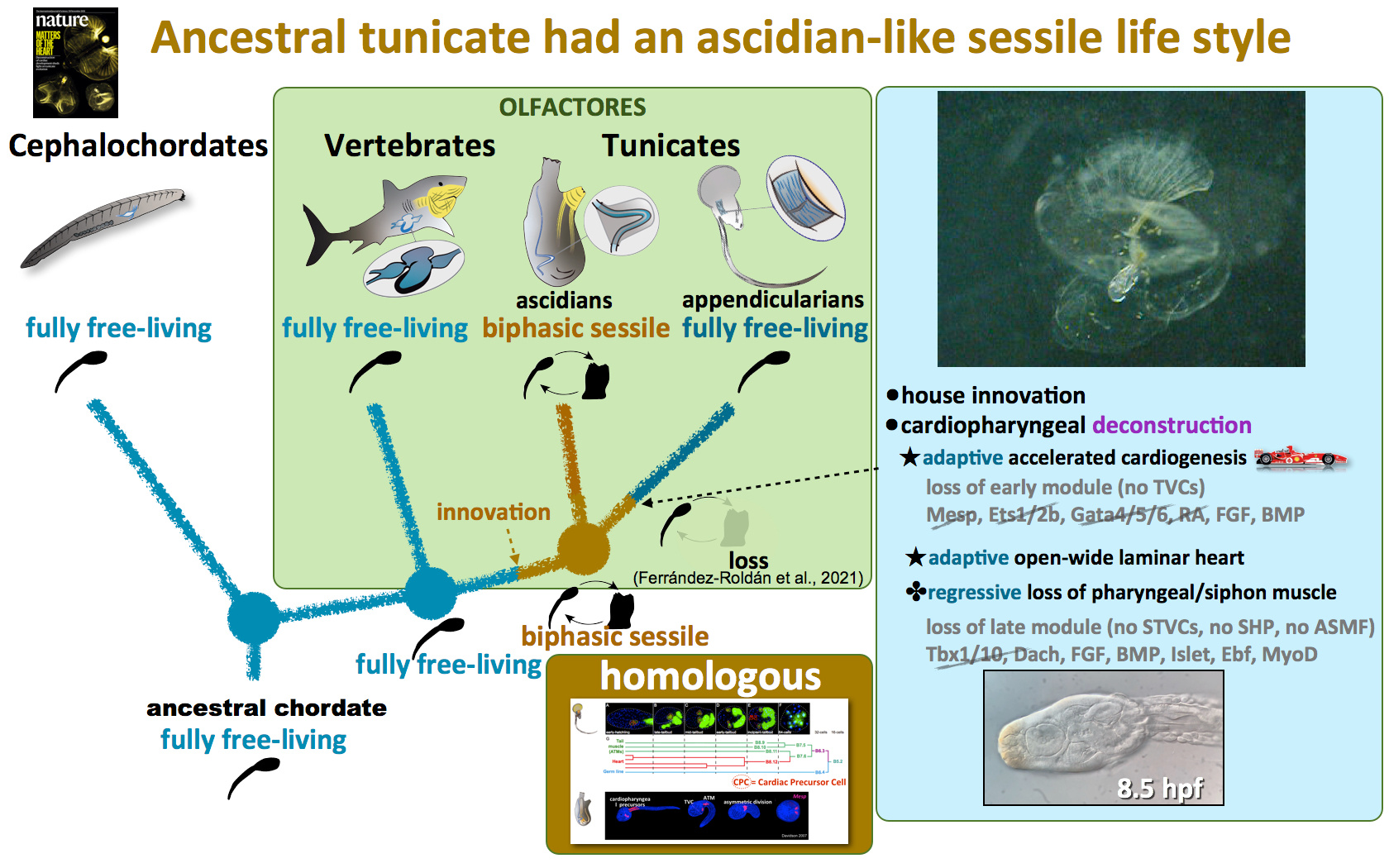
The evolutionary scenario of chordates
The discovery that tunicates are the sister group of vertebrates, and therefore that the branching of cephalochordates is basal within chordates2–5, provided a novel modern view of the last common ancestor of chordates as a free-living organism, in contrast to the traditional view proposed by Garstang (1928)6 in which it had a sessile ascidian-like adult lifestyle. This novel view provided a new phylogenetic framework to investigate the enigmatic origin of sessility in tunicates. Moreover, it brought renewed attention to appendicularian tunicates, whose complete free-living style could parsimoniously represent the ancestral tunicate condition considering their most accepted position as the sister group of the remaining tunicates.
The Oikopleura model in EcoEvoDevo
During the last decade, the research in our laboratory has focused on the study of the appendicularian tunicate Oikopleura dioica because this tiny zooplanktonic creature, in contrast to ascidians, does not suffer a drastic metamorphosis to become a sessile adult, but retain its chordate body plan and a pelagic free-swimming life style throughout their entire life. This unique characteristic of appendicularians can be useful as an EvoDevo model to better understand the ancestral condition of all tunicates as well as how tunicates and vertebrates diverged after the split of their lineages from a common relative predecessor. Appendicularians’ unique way of feeding with the house makes these organisms very interesting to also be studied as an EcoEvoDevo model in the context of climate emergency7 (for further reading see this other post in the blog of Ecology and Evolution: “Are embryos ready for climate change?”). The house is a filtering jelly device secreted by the oikoblastic epithelial of the trunk that efficiently traps microalgae from broad range of sizes, making thus a short cut in the trophic web and accumulating lots of organic matter that contributes to carbon cycle by sinking in the oceans. The house (oikos in greek) is the key evolutionary innovation that characterizes the free swimming style of this group of animals.
The Oikopleura model for gene loss
Our interest in studying O. dioica as an evodevo model to understand the impact of gene loss began when we suddenly transformed our frustration of not finding any of our favorite genes into the central question of our research by realizing that the reason why we were not able to find so many genes surprisingly was because they had been lost8. We started to become intrigued on how was possible that some organs could develop even in the absence of genes that were thought to be essential in other chordate species? That was the beginning of what we referred as the “evodevo inverse paradox”, which highlighted the fact that similar phenotypic features masked important genetic differences, in many cases due to gene losses9.
The EvoDevoGenomeUB lab in Barcelona
The loss of retinoic acid (RA) signaling was probably the first surprising discovery of gene losses in O. dioica. The work of Josep Martí-Solans, the first PhD in our lab when we started in 2010, and who played a fundamental role in the setting up of our animal facility in Barcelona10, described the dismantling and loss of function of the RA pathway11 confirming previous results obtained in the University of Oregon12. The loss of RA signaling in O. dioica contrasted with its conserved pivotal roles in other chordates in many essential processes such as anteroposterior-axial patterning, neurogenesis, visual system development, gonadogenesis, adult tissue homeostasis and cardiogenesis among many others. Past lab members, including many undergrads and master students, passed through the lab contributing to investigate how the loss of RA signaling could have affected the evolution of other signaling pathways such as Fgf and Wnt, which normally counteract RA in many developmental processes13. It was 2014-2015, when we were seeking for a particular developmental gene regulatory network as a case study in which we could focus and analyze in depth the impact of gene loss. It was at that time when Alfonso Ferrández-Roldán had recently joined our group as an undergrad @BiologiaUB and enrolled in our @MasterGeneUB program, when we finally choose heart development as the case study for his Ph.D. project to investigate the impact of gene loss on the evolution of the mechanisms of development in appendicularians.
This decision was motivated by two coincidental reasons. The first reason was the publication of the novel hypothesis “a new heart for a new head” about the evolution of lifestyle in chordates and the origin of vertebrates by Rui Diogo et al.14. This novel hypothesis, in addition to the relevance of the evolution of a new head with placode and neural crest derivatives as proposed by Gans and Northcutt15, pointed to the development of a chambered heart and elaborated branchiomeric muscles as key evolutionary innovations that facilitated the transition from the peaceful filter-feeder lifestyle of ancestral olfactores to the voracious predatory lifestyle of vertebrates. That hypothesis suggested that investigating heart development in O. dioica could provide new insights into the evolution of lifestyle in tunicates.
The second reason appeared one night watching the Catalan news on TV3 about a discovery by a research group in North of Spain of a novel sarcomeric cardiac gene, Filamin C16, which could be used for the diagnostic of hypertrophic cardiomyopathy, an alteration that is one of the main causes of sudden deaths with tragic consequences in young athletes. That day, we came with the idea that the simplicity of the heart of O. dioica (the adult heart only has 6 myocardial cells) could also become a useful new model animal to better understand the genetic bases of cardiomyopathies. Coincidentally that year there was a Catalan crowdfunding call organized by “La marató de TV3” dedicated to heart disease, which encouraged us to start a new endeavor with some applied focus towards biomedicine. Lack of enough funding for research, which is a chronic problem in Spain, specially at that time still under the effects of the post economic crisis, was seriously threatening the survival of our laboratory after two tough years with no funding. In an attempt to look for alternative sources of funding, we team up with Dr. Eliécer Coto, an expert in cardiomyopathy genetics from the Hospital Universitario Central de Asturias and one of the coauthors of the Filamin C article, and we applied for “La marató” and many other governmental and non-governamental calls. Despite initially none of proposals being awarded, all the brainstorming and efforts we put in the writing of those grant proposals about heart were the fuel and inspiration for the last two national projects from Ministerio de Ciencia y Innovación that have finally been funded.
The cardiopharyngeal homology in Olfactores
At the beginning of Alfonso’s PhD project, the information about O. dioica’s heart was scarce and limited to some descriptions made by Salensky in the 1900s consisting on a laminar chamberless cardiac structure simply made of two layers (i.e. the myocardium and pericardium) that propelled the hemolymph by beating against the stomach wall. Alfonso first challenge was to describe a modern developmental atlas of the heart of O. dioica and to provide evidence that the laminar heart of O. dioica and the cylindrical heart of ascidians, despite their striking morphological differences, were indeed homologous. Alfonso showed that O. dioica’s first cardiac progenitor cells (CPC) share the same lineage than the most anterior axial cells of the tail, a common feature also shared with ascidians and vertebrates, providing, therefore, further evidence of a conserved cardiopharyngeal program already present in the last common ancestor of Olfactores (i.e. tunicates + vertebrates). Thus, this work provided evidence supporting the homology between the hearts of tunicates and vertebrates, as well as between their pharyngeal muscles in the tunicate trunk and the head in vertebrates.
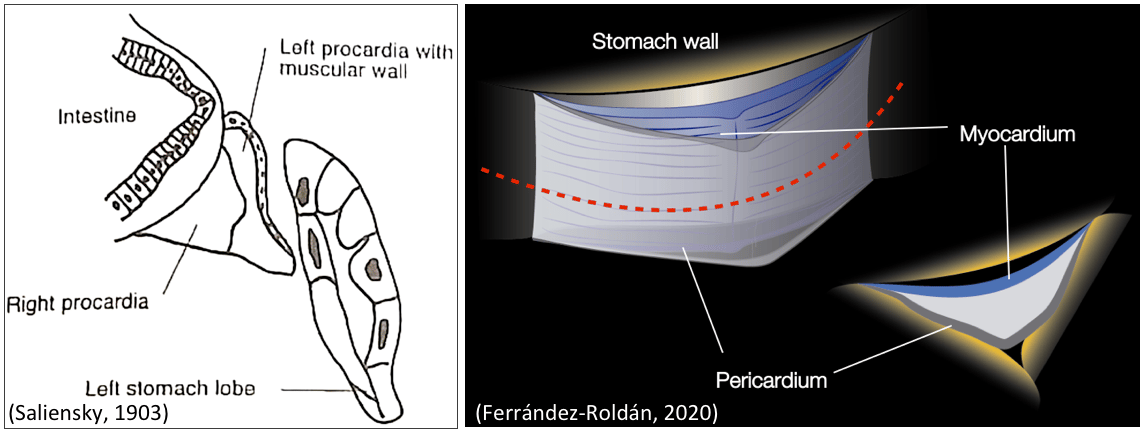
Representation of the laminar heart of O. dioica as represented by Saliensky (1903) and Alfonso Ferrández-Roldán (2020)
The deconstruction of cardiopharyngeal gene regulatory network
One of the most striking results from Alfonso’s thesis is the discovery of massive ancestral losses of genes and cardiac subfunctions at the base of the appendicularian clade. The absence of Mesp was probably the most unexpected loss, since this is considered a master cardiac gene essential for the development of pre-cardiac multipotent precursors in ascidians and vertebrates. Overall, these massive losses, including the absence of Fgf and Bmp signaling for cardiac determination, pointed to a “deconstruction” of two modules of the cardiopharyngeal gene regulatory network that in ascidians are related to early and late multipotent cells involved in lineage fate determination towards the first and the secondary heart fields, as well as towards siphon muscles.
The concept of ‘deconstruction’ in Evolutionary and Developmental Biology
The term “deconstruction”, originally coined in Philosophy and later applied in disciplines such as Literature, Architecture, Fashion, Cinema and even in Cookery by the revolutionary Catalan top chef Ferran Adrià, is not synonymous with destruction, but it generally refers to the process of dismantling or breaking apart elements that traditionally are combined, and whose analysis facilitate the recognition of structural modules. Our work shows how the evolutionary deconstruction by co-elimination of genes and subfunctions during evolution highlights the modular organization of gene regulatory networks. Thus, the characterization of the “lossosome” (catalogue of gene losses in a specific phylogenetic context)17 can be a useful tool to decipher the architecture and evolution of gene regulatory networks and their relation with the origin of evolutionary innovations and adaptations in different species.
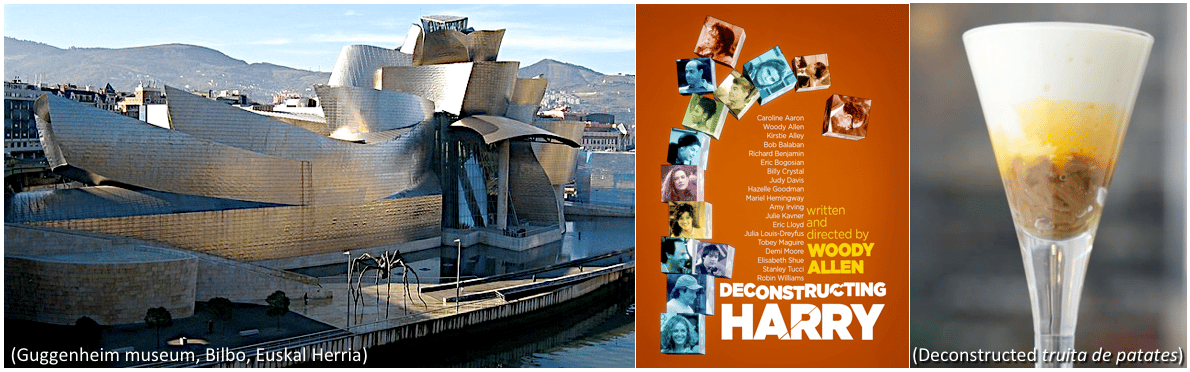
The concept of 'deconstruction' was coined in the field of philosophy, and later applied to many other areas such as literature, fashion, architecture (e.g. the Guggenheim museum in Bilbo), cinema (e.g. Deconstructing Harry by Woody Allen), Cookery (e.g. the revolutionary deconstructed truita de patates –potato omelet– innovated by Ferran Adrià), and now in our work to the field of Evolutionary and Developmental Biology.
Appendicularian adaptive deconstruction and ancestral sessility of tunicates
Our work proposes an evolutionary scenario in which the deconstruction of the cardiopharyngeal gene regulatory network can be connected with three evolutionary innovations of the appendicularians : i) an accelerated cardiogenesis, ii) the formation of an open-wide laminar heart, and iii) the loss of siphon muscle. These evolutionary innovations can be related to the transition from an ancestral sessile ascidian-like adult lifestyle to the pelagic fully free lifestyle of appendicularians.
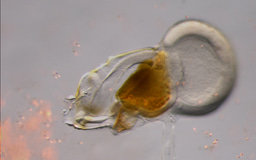
The deconstruction of the “early module” and the absence of multipotent cardiopharyngeal cell states has led to an earlier activation of the cardiac kernel (e.g. Nk4 and Hand1/2). This early activation has driven a more accelerated cardiogenesis in appendicularians than in ascidians, which can be interpreted as an adaptation to the faster development in appendicularians than in ascidians. In ascidians, heart development is not completed until several days after the metamorphosis, time at which larva become sessile adults. In appendicularians, conversely, heart beating begins as soon as 8.5 hours post fertilization, being fully functional when juveniles inflate the first house and begin their active free-swimming lifestyle.
The deconstruction of the “late module” can be connected with the loss of the secondary multipotent cardiopharyngeal cell states. This loss correlates with the absence of second heart field, which plausibly can be related to the reorganization of the heart from a tubular into an open-wide laminar structure as an adaptation that favors hemolymph circulation propelled by the movements of the tail. This loss also correlates with the loss of any pharyngeal muscle in the trunk that could be homolog to siphon muscle ascidians. This loss is likely the result of regressive evolution since the functions related to siphon contraction became useless upon the innovation of the house.
In conclusion, our results support an evolutionary adaptive scenario in which the deconstruction of cardiopharyngeal GRN could have contributed to the loss of features that characterize ascidian-like sessile lifestyle, and the acquisition of novel features that enabled its transition to a pelagic free-living active style connected to the innovation of the house.
The scientific pots
It is also important to highlight that the story behind the paper has a long past before we set our lab in Barcelona. Scientists and labs can be considered as "plant pots" into which mentors, colleagues and past lab members have been adding seeds, soil, nutrients, water … until constellations align into the adequate conditions that make all the components to bloom into new breakthroughs and ideas. Behind this paper there is an endless list of people that have contributed to our scientific pot to make it possible, and among many, I would like to highlight two of the most influential persons to whom I’d like to thank because without them this article would not have been possible.
Roser Gonzàlez-Duarte, full professor in the Department of Genetics of the UB was the person who accepted me as an undergrad in her group, a turning point in my life. Roser became my PhD supervisor together with Ricard Albalat, constructing with the later a friendship beyond the lab, and becoming an inseparable team devoted to endless discussions about Evolution. Roser had the clairvoyant inspiration of introducing me into the field of chordate EvoDevo at the time in which the EvoDevo school of Barcelona was being forged by Jordi Garcia-Fernàndez following Jaume Baguñá’s legacy. Among the many teachings that Roser seeded into my pot, there are two that have always accompanied my projects during all these years: aiming for the highest quality standard as the signature of our work, and humbleness as the pillar sustaining our achievements.
John Postlethwait, full professor in the Institute of Neurosciences in the University of Oregon, who hosted me as postdoctoral associate researcher for near 8 years. John did not only open to me the doors to the world of Oikopleura allowing me to work with Susie Bassham, the person who pioneered the study of this organism in EvoDevo, but John also contributed with many ingredients into my scientific pot that have been crucial. With his extraordinary capacity to provide a global perspective about everything, John taught me how Science can be deeply enjoyed just for the genuine sake of curiosity, and importantly, he taught me how to never be afraid to ask big questions in Biology. John’s passion for running, endurance and constancy have probably been an inspiration for me to overcome the many obstacles that we have had to face to make this work possible.
The results of this article, therefore, would have not been possible without the many past contributions to the scientific pot of our lab, the incredibly kind research EvoDevo community of chordates and tunicates, and specially thanks to the many former members of the lab. While writing this post, we just learnt that from near 130 papers published in Nature by researchers of the University of Barcelona, there are less than five articles, including ours, in which all authors are exclusively from the University of Barcelona. Our reserach team, however, it is not very different from many others we have around in Catalan public universities, which count on many talented people plenty of potential, but all of us having in common the gigantic efforts we have to constantly made to progress through this hurdle race to find enough funding to maintain our labs up and running. The results of this work, therefore, should be taken as an incentive for governmental funding agencies to increase research budgets so the pots of many valuable labs and researchers in public universities performing basic research can also bloom to benefit our society and to face the imminent coming challenges such as planet sustainability, preservation of biodiversity, pandemics and climate emergency. It is time to invest in basic research, to implement a solid system that allow scientists (specially the many well prepared students that are ready to become researchers) to work in suitable conditions, paying special attention to public universities.
To stay tuned with the research of our lab you can follow us in Twitter @evodevogenomeUB and visit our web page https://evodevogenomics-unibarcelona.weebly.com/
- Albalat, R. & Cañestro, C. Evolution by gene loss. Nat. Rev. Genet. 17, 379–391 (2016).
- Delsuc, F., Brinkmann, H., Chourrout, D. & Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968 (2006).
- Bourlat, S. J. et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 444, 85–88 (2006).
- Satoh, N. Chapter 2 - Hypotheses on Chordate Origins. in Chordate Origins and Evolution (ed. Satoh, N.) 17–30 (Academic Press, 2016). doi:https://doi.org/10.1016/B978-0-12-802996-1.00002-X.
- Gee, H. Across the Bridge: Understanding the Origin of the Vertebrates. (University of Chicago Press, 2018).
- Garstang, W. The morphology of the Tunicata, and its bearings on the phylogeny of the chrodata. Quar. J. Micr. Sci. 72, 51–186 (1928).
- Torres-Águila, N. P. et al. Diatom bloom-derived biotoxins cause aberrant development and gene expression in the appendicularian chordate Oikopleura dioica. Commun. Biol. 1, (2018).
- Ferrández-Roldán, A., Martí-Solans, J., Cañestro, C. & Albalat, R. Oikopleura dioica: An Emergent Chordate Model to Study the Impact of Gene Loss on the Evolution of the Mechanisms of Development. in Evo-Devo: Non-model Species in Cell and Developmental Biology. (eds. Tworzydlo, W. & Bilinski, S.) vol. 68 63–105 (Springer, Cham, 2019).
- Cañestro, C. et al. Evolutionary developmental biology and genomics. Nat. Rev. Genet. 8, 932–42 (2007).
- Martí-Solans, J. et al. Oikopleura dioica culturing made easy: A Low-Cost facility for an emerging animal model in EvoDevo. Genesis 53, 183–193 (2015).
- Martí-Solans, J. et al. Coelimination and Survival in Gene Network Evolution: Dismantling the RA-Signaling in a Chordate. Mol. Biol. Evol. 33, 2401–2416 (2016).
- Cañestro, C. & Postlethwait, J. H. Development of a chordate anterior-posterior axis without classical retinoic acid signaling. Dev Biol 305, 522–538 (2007).
- Martí-Solans, J. et al. Massive Gene Loss and Function Shuffling in Appendicularians Stretch the Boundaries of Chordate Wnt Family Evolution. Front. cell Dev. Biol. 9, 700827 (2021).
- Diogo, R. et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520, 466–473 (2015).
- Gans, C. & Northcutt, R. G. Neural crest and the origin of vertebrates: a new head. Science (80-. ). 220, 268–274 (1983).
- Valdés-Mas, R. et al. Mutations in filamin C cause a new form of familial hypertrophic cardiomyopathy. Nat. Commun. 5, 5326 (2014).
- Cañestro, C. & Roncalli, V. Gene losses did not stop the evolution of big brains. Elife 7, (2018).
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
The link given for sharing (https://go.nature.com/3D3R9EP) does not work.
Dear Susanne,
Please get in touch with us at communities@nature.com with your query.
Best wishes,
Eve
Hi Susanne, did it finally work? (it does for me)
That link was to share the story, and this other one is to have free access to the article https://t.co/4rJA2MURKX?amp=1