Deep tissue physiology monitoring enabled by a wireless ultrasound technology
Published in Bioengineering & Biotechnology

Deep tissue signals often have a stronger correlation with critical physiological processes, such as circulation and respiration1. Medical ultrasound is a highly effective tool for capturing these deep tissue signals. However, for most people, this technology remains inaccessible in their daily lives. The ultrasound tests are limited in hospitals, where costly machines and experienced clinicians play a key role.
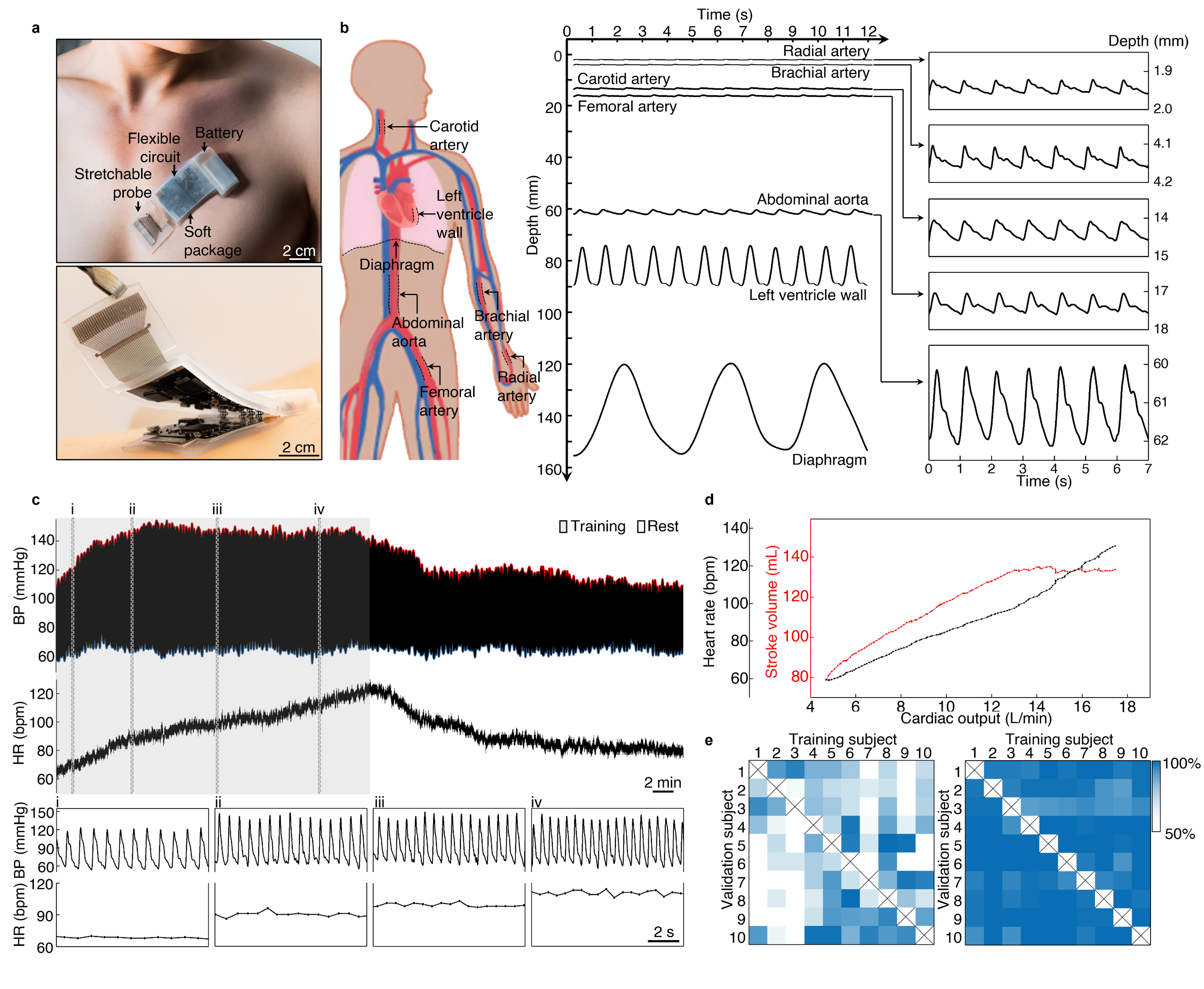
Recognizing the challenges posed by the limited availability of ultrasound technology in non-clinical settings, we set out to develop a solution. We aimed to design a fully integrated, autonomous ultrasonic system-on-patch. This patch incorporates both ultrasonic probe and wireless control electronics in a soft, wearable format (Fig. 1a). Not only is it cost-effective, but it also eliminates the need for a trained operator. The deep tissue signals captured from the subject are conditioned and preprocessed on-patch, then wirelessly transferred to a backend receiver, where they are analyzed by a customized machine learning algorithm.
We made this ultrasonic system-on-patch capable of capturing a broad range of deep tissue signals, including arterial pulses, ventricular contractions, and diaphragm excursions (Fig. 1b), which contain rich physiological information and diagnostic value. From arterial pulse waveforms, the critical parameters associated with cardiovascular diseases can be derived, such as heart rate, blood pressure, and arterial compliance. From ventricular contractions, the diameter change of the heart chamber during cardiac cycles can be derived, which is a quantitative measure of heart function. The diaphragmatic excursion is a surrogate for lung function, which can be used to characterize typical respiratory volumes and diagnose respiratory issues, such as airway obstruction or lung capacity restriction.
To further demonstrate the patch's capabilities, we conducted a study on a participant during exercise. The participant performed 30 min continuous cycling followed by 30 min rest. We recorded the carotid blood pressure waveform and heart rate while the participant moves freely (Fig. 1c). We also estimated the stroke volume from the pressure waveforms using a pulse contour method2. The cardiac output is then calculated as the product of stroke volume and heart rate. Our results showed that as exercise intensity increased, the measured cardiac output also increased, with the heart rate following suit. The stroke volume increases initially and then plateaued due to limited ventricle size (Fig. 1d).
On the backend, the machine learning algorithm used for autonomous data processing appeared to be flawless. However, a common pitfall is the poor generalizability of the algorithm. Many algorithms struggle to accept data with new features that deviate from the training dataset, and so was ours. We initially characterized the generalizability through a ten-subject cross-validation test. We trained the model on each subject and then validated it on the nine other subjects. Unfortunately, the results were unacceptable, with the model only achieving an average accuracy of 63.23% on new subjects (Fig. 1e left). To address this generalization problem, we leveraged the domain adaptation technique3 developed by computer scientists to minimize the data discrepancies between subjects. With minimal efforts in data collection from the new subject, the model could adapt to the new subject with high accuracy (Fig. 1e right). By addressing the generalization problem, we can ensure that our patch technology is reliable for a wide range of individuals.
In summary, the development of the fully integrated ultrasound patch represents a significant breakthrough in making ultrasound technology accessible for daily healthcare scenarios. With its wireless connectivity, it eliminates the need for cumbersome wires and allows for greater mobility during data acquisition. The real-time, machine learning-based algorithms automate data processing and analysis, freeing up valuable time and resources for clinicians. Together, these innovations pave the way for continuous surveillance of deep tissue physiology toward internet-of-medical-things.
This study entitled " A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects " has been published in Nature Biotechnology (https://www.nature.com/articles/s41587-023-01800-0).
Reference
1 Lin, M., Hu, H., Zhou, S. & Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 7, 850–869, doi:10.1038/s41578-022-00427-y (2022).
2 Scolletta, S., Biagioli, B. & Giomarelli, P. in Anaesthesia, Pain, Intensive Care and Emergency APICE 225-236 (Springer, 2007).
3 Morerio, P., Cavazza, J. & Murino, V. Minimal-entropy correlation alignment for unsupervised deep domain adaptation. in International Conference on Learning Representations (2018).
Follow the Topic
-
Nature Biotechnology

A monthly journal covering the science and business of biotechnology, with new concepts in technology/methodology of relevance to the biological, biomedical, agricultural and environmental sciences.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in