Degradation of water soluble poly(vinyl alcohol) with cavitation: foundations for microplastics
Published in Earth & Environment

Plastic pollution has become the subject of significant scientific attention. This is due to the now apparent ubiquity of plastics in the environment, their large and increasing quantities, the diversity of sources, the types of the materials, and the largely unknown fate and long-term effects. Concern has shifted from the initial interest in macro plastic litter, to all forms of microplastics and the current "search" for nanoplastics - particles smaller than 1 µm ranging into the nanometer scale. The same concerns should apply to water-soluble synthetic polymers, especially full carbon- backbone polymers. Their solubility makes them invisible, however they can persist in the environment and cause unwanted consequences.
Our research group from the University of Ljubljana (SI) and the National Institute of Chemistry (SI) addressed the degradation of water-soluble PVOH, which is widely used in the textile and paper industries, beside that many of us use it every day in convenient detergent pods in washing machines that miraculously disappear when put in water. According to some estimates, 7000 tons of this material is released through wastewater treatment plants in the U.S. alone because they are not equipped to treat or retain it. As global industrial demand is geared toward greater consumption of polymers, concentrations of these materials in the environment will only increase.
Cavitation, a physical phenomenon first studied for its destructive effects on ship propellers, noise generation on submarines, or reduced performance of water turbines and water pumps, is nowadays widely utilized in many industrial processes. The phenomenon associated with the growth and collapse of small vapor bubbles in a liquid due to a local pressure drop can be used for a variety of processes. During collapse, all energy is concentrated in an extremely small spatiotemporal region, creating extreme conditions in the form of shockwaves, shear, microjets, and potentially extreme temperatures. These properties are used in cleaning hard-to-reach surfaces, medical applications, food and pharmaceutical industries, and wastewater treatment. Since cavitation conditions can form a "perfect" microchemical reactor for dissociation of water molecules into preferred OH radicals and can cause chemical consequences - oxidation - in addition to mechanical effects, cavitation belongs to the class of advanced oxidation processes.
In our research, we focused on the degradation of water-soluble PVOH by acoustic and hydrodynamic cavitation. Although the nature of a single cavitation bubble is more or less the same for acoustic or hydrodynamic induction, there are specific differences between the two types. The first type, induced with ultrasonic waves, can be more pronounced in its power concentration, while the second - hydrodynamic cavitation - can be more easily manipulated and scaled for real applications. By comparing the two types, we gained detailed insights into the mechanisms of PVOH degradation. We discovered that acoustic cavitation predominantly causes chain scission through mechanical effects, while chemical effects also play an important role in hydrodynamic cavitation. In particular, when the length of polymer chains approaches oligomers and monomers, mechanical events can no longer affect them, while chemical effects are insensitive to their length.
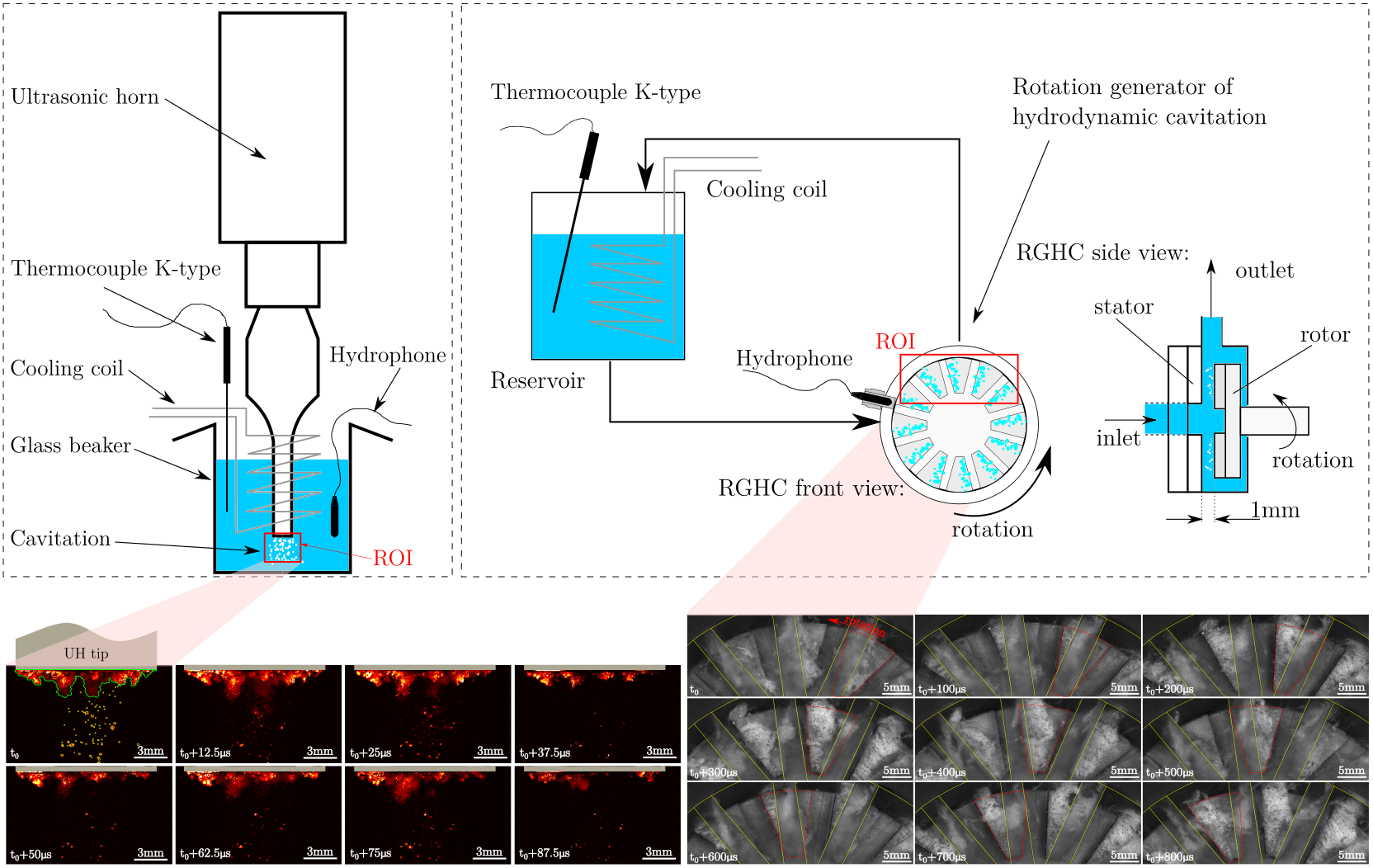
Figure: Schematic presentation of laboratory test rigs with acoustic cavitation (left) and hydrodynamic cavitation (right). Below is a sequence of images of cavitation formation under the tip of the ultrasonic horn (left) and in the rotating generator of hydrodynamic cavitation (right). Image author: Dr. Martin Petkovšek
The degradation of PVOH to very short oligomer chains achieved by hydrodynamic cavitation is an important achievement, since oligomers with low molar mass are more easily degraded in nature. As shown by the FTIR analysis, the oligomers were highly oxidized, which is generally a factor that increases reactivity and promotes biodegradation. This study can serve as a starting point for the degradation of other polymers that may be more harmful and difficult to degrade, such as microplastics. However, before the technology can be considered safe and used on a pilot or industrial scale, TOC and ecotoxicity studies should be conducted. These analyzes would show in more detail the extent to which cavitation can mineralize PVOH and confirm that no undesirable or even toxic products have been generated.
Follow the Topic
-
npj Clean Water

This journal publishes high-quality papers which report cutting-edge science, technology, application, policy and societal issues that contribute towards a more sustainable supply of clean water.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advancing Artificial Intelligence Innovations for Resilient and Sustainable Clean Water: Novel Methodologies and Case Studies
Publishing Model: Open Access
Deadline: Feb 28, 2026
Advances in Smart Water Systems: From Source to Tap and Beyond
Publishing Model: Open Access
Deadline: Feb 28, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in