Dense inorganic electrolyte particles as a lever to promote composite electrolyte conductivity.
Published in Materials

James A. Isaac, Didier Devaux, Renaud Bouchet#
Traditionally, liquid electrolytes (LEs) have been used for lithium ion batteries due to their high conductivity and good wettability of electrodes, but are far from ideal in terms of safety due to their flammable nature. LEs inability to suppress dendrite formation (causing short circuits and fire hazards upon cycling) also prevents the use of Li metal, the holy grail of negative electrodes. Consequently, there has been an increased drive towards using solid state electrolytes as these could both improve safety and suppress dendrite formation.
There are two major classes of solid state electrolytes: solid polymers (SPE) and ceramics (CE). SPE are advantageous in terms of their soft mechanical properties promising low cost processing such as solvent-free extrusion, and intimate contact with electrodes. However, the low SPE conductivity hinder their practical application at room temperature. CEs have greater ionic conductivity and thermal stability, but their shaping requires high temperature and/or pressure leading to brittle materials. Intimate Li/ceramic interfaces are difficult to form and maintain during the volume change of lithium upon cycling (Li platting/striping), generating delamination and cell failure and/or dendrite formation. Composite electrolytes comprising of dispersing ceramic particles in a polymer matrix seems a promising compromise to benefit from the advantages of each.
One would expect the conductivity of a SPE/CE composite electrolyte to have a conductivity in between that of SPE and CE, but the results reported in the literature are diverse, unexpected and inconsistent. For very similar SPE/CE couples, certain groups report an increase in conductivity of several orders of magnitude when the CE is added to the SPE, whereas others simply observe a decrease in conductivity. Certain cases have even been reported where the composite electrolyte has a higher conductivity than each of its components. Evidently, the factors driving the conductivity of dispersed CEs in conducting organic matrices are not clear, and a physical description of the effective conductivity is thus highly desired. Importantly, the impact of both the polymer electrolyte reactivity with the ceramic particles and the particle microstructure have been disregarded.
In this manuscript a broad series of composites are screened with 7 different CEs, 4 LEs (modelling SPEs) and salt concentration varied over 3 orders of magnitude. A LE is used instead of a SPE so that artefacts due to reactivity between the CE and SPE/LE, which would artificially increase the conductivity of the composite, can be eliminated (once the conductivity of the composite has been measured the LE and CE powder can be separated and the conductivity of the LE re-measured).
Here we demonstrate that while CEs such as LATP, LAGP, LLZO, and LLTO typically have a bulk (grain) ionic conductivity of 10-3 to 10-4 S.cm-1 in the form of a dense pellet, their conductivity when dispersed in a LE is determined by their microstructure (see Scheme 1). Porous/agglomerated CE particles behave as insulating. This can be rationalised by considering that in an agglomerate, the point contacts between grains render the structure highly resistive. Conversely the effective conductivity of a dense particle in suspension remains the same as that of a dense pellet. In practical terms, the addition of dense particles increases the conductivity of the composite when the conductivity of the CE is higher than that of the SPE/LE (normally the case for CE/SPE couples), whereas the addition of porous particles always decreases the conductivity (see Scheme 1).
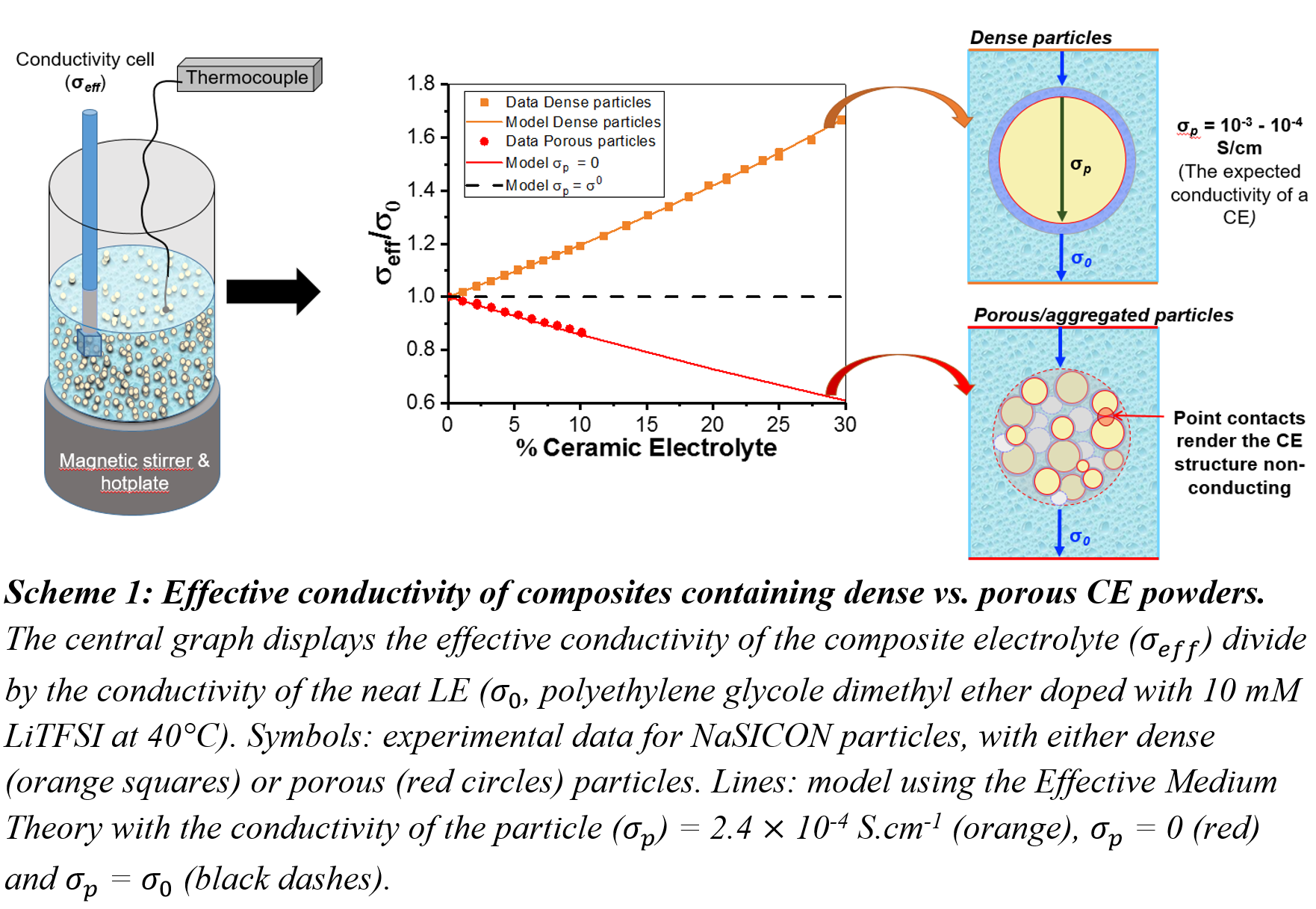
All of the experimental data herein can be modelled with an effective medium theory, with excellent correlation, enabling predictions for any composite having knowledge of i) microstructure and ii) the conductivity of each component in the composite. Predictions using the Effective Medium Theory suggest that gains in conductivity can be obtained using dense ceramic particles when the particle has a higher conductivity than the LE/SPE, however the continuous phase (LE/SPE) dominates the conductivity properties. The gains in conductivity are of the order of a factor of two at 20 vol% CE rising to a factor of 8 at 70 vol% CE compared to the neat polymer, in the case of a CE that is infinitely more conducting than the LE/SPE. Thus a continuous phase (LE/SPE) with a good conductivity is necessary to make an effective composite electrolyte.
Interestingly out of all the commercial CE powders on the market, only one supplier of dense CE particles was found. The results presented within this work have clear implications for both CE suppliers and for those working in the domain of composite electrolytes, as it is likely that most groups will be using aggregated CE powders that behave no better than insulating ceramics in terms of their conductivity properties.
Within this manuscript, some of the rules that govern the conductivity of composite electrolytes are uncovered. The results presented should guide researchers and suppliers towards superior composite electrolytes.
Follow the Topic
-
Nature Materials

A monthly multi-disciplinary journal that brings together cutting-edge research across the entire spectrum of materials science and engineering, including applied and fundamental aspects of the synthesis/processing, structure/composition, properties and performance of materials.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in