Designing Better Hydrogen Catalysts—One Atom at a Time
Hydrogen is often hailed as the fuel of the future—but unlocking its full potential requires catalysts that are not only efficient but also affordable and scalable. Platinum has long been the gold standard for the hydrogen evolution reaction (HER), but its scarcity and cost pose major barriers to widespread adoption.
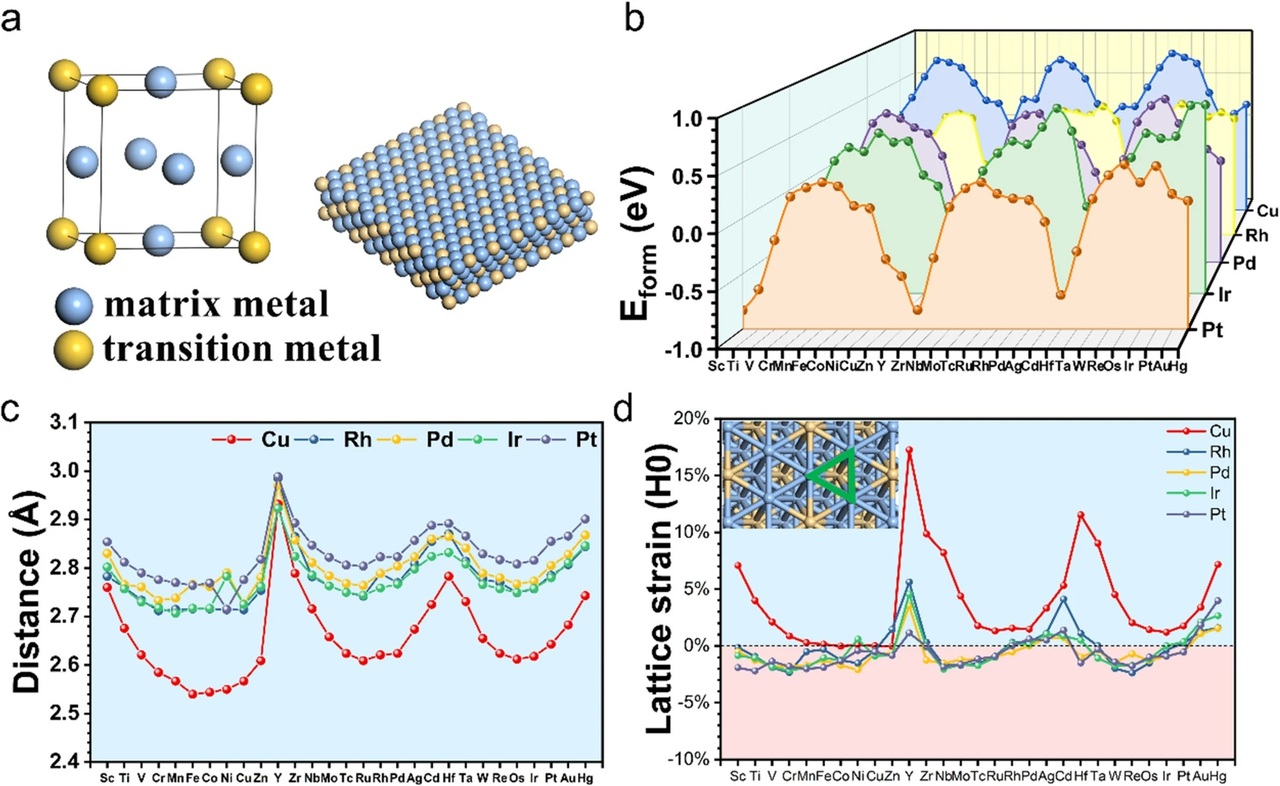
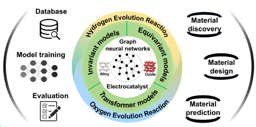
In our latest study, we set out to explore whether intermetallic compounds (IMCs)—specifically those doped with transition metals—could offer a viable alternative. Using density functional theory (DFT) calculations, we systematically screened 145 L1₂-type IMCs, combining noble metals like Rh, Pd, Ir, and Pt with a wide range of 3d, 4d, and 5d transition metals.
What we found was both surprising and exciting.
Key Insights
-
Noble metals still matter—but not in the way we thought.
Over 70% of the optimal active sites were dominated by noble metal atoms, while the doped transition metals primarily acted as electronic modulators, tuning the catalytic behavior without directly participating in the reaction. -
The volcano-shaped trend is predictive.
We observed a volcano-shaped relationship between hydrogen adsorption free energy and catalytic activity, confirming the Sabatier principle and offering a roadmap for identifying high-performance materials. -
14 IMC catalysts outperformed platinum.
Among the 145 candidates, 14 IMCs—including Pt₃H, Pd₃Zr, and Rh₃Cr—showed superior HER activity and thermal stability compared to conventional Pt catalysts. -
The d-band center theory holds up—even in complex systems.
Our results extend the applicability of d-band center theory to multi-metal environments, revealing how orbital hybridization and lattice strain influence HER performance at the atomic level.
This work is more than a computational exercise—it’s a step toward rational catalyst design, where atomic-scale insights guide the development of materials that are not only effective, but also economically viable.
We hope our findings will inspire further experimental validation and accelerate the transition to a hydrogen-powered future.
Follow the Topic
-
Catal

Catal is an open access journal covering full spectrum of catalysis critical advances. From biocatalysts to heterogeneous catalysts, it integrates fundamental and applied sciences. Catal offers a primary platform for researchers and practitioners in the field.
Related Collections
With Collections, you can get published faster and increase your visibility.
Bio-Catalysis in Circular Bioeconomy and Green Chemistry
This collection emphasizes the role of bio-catalysis in advancing the circular bioeconomy, focusing on enzymatic transformations and eco-friendly processes that valorize renewable feedstocks. Contributions should highlight innovative applications of bio-catalysis in waste-to-value systems, biorefineries, and green chemical synthesis.
Catal invites research articles, reviews and reports on the topic of the development of enzymes, metabolic engineering, and integration of bio-catalysis into industrial processes, aiming to reduce dependency on fossil-based resources and promote sustainable practices.
Publishing Model: Open Access
Deadline: Mar 31, 2026
Nanocatalysis and Thermocatalysis in Precision Chemical Synthesis
This collection, hosted by Catal, highlights the intersection of nanocatalysis and thermocatalysis in precision chemical synthesis. It aims to disseminate cutting-edge research that drives innovation in catalytic materials, selective processes, and reaction pathways, fostering advancements in the production of fine chemicals and specialty compounds. Aligned with Catal's mission to prioritize impactful catalytic applications, this collection welcomes contributions from established and early-career researchers that advance both theoretical and applied catalysis.
The collection embraces the breadth of Catal’s coverage, including topics such as nanostructured catalysts, thermocatalytic processes, and advanced synthesis strategies. Contributions may explore catalytic mechanisms, computational modeling, or experimental breakthroughs, offering insights into scalable industrial applications and fundamental research. Articles types—original research, reviews, perspectives, and analyses—are all encouraged, ensuring a diverse platform for sharing high-impact advancements in catalysis.
Publishing Model: Open Access
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in