Designing Bugs Against Cancer
Published in Bioengineering & Biotechnology

In 1891, Dr. William B. Coley successfully treated cancer by injecting streptococcus into a patient’s tumor1. Since then, we have learned a lot about the role of the immune system but the search for methods to stimulate the innate immune response to cancer continues. Several groups have explored the use of attenuated pathogenic bacteria as cancer treatments. These approaches have been limited as the strains used have either failed to demonstrate robust efficacy or exhibited unacceptable side effects2. We asked whether synthetic biology would allow us to take non-pathogenic bacteria and engineer specific attributes including efficacy and safety features that would extend William Coley’s work as a new way to build living medicines.
A non-pathogenic bacteria E coli Nissle 1917 (EcN) is highly engineerable, easily scalable for manufacturing, and has a long history of safe use in humans as a probiotic3. We have pioneered the development, manufacturing, and clinical translation of E coli Nissle based medicines4 for metabolic diseases5,6, inflammation, and cancer. Using synthetic biology techniques, we designed genetic circuits that enable the bacteria to sense and modulate a disease. Our approach is differentiated from many previous efforts to engineer bacteria, which while highly creative, have failed to include design elements important to translate from bench-to-bedside.
In our most recent study in Nature Communications we develop an immunotherapeutic EcN strain, SYNB1891, designed for the treatment of difficult to treat cancers and describe the implementation of design elements critical for its use as a human therapeutic.
Our team assessed the capabilities of EcN as a potential cancer therapeutic including its ability to survive in tumors and evaluated an array of relevant immunomodulatory molecules and reusable parts that could be expressed in EcN (see Figure 1).
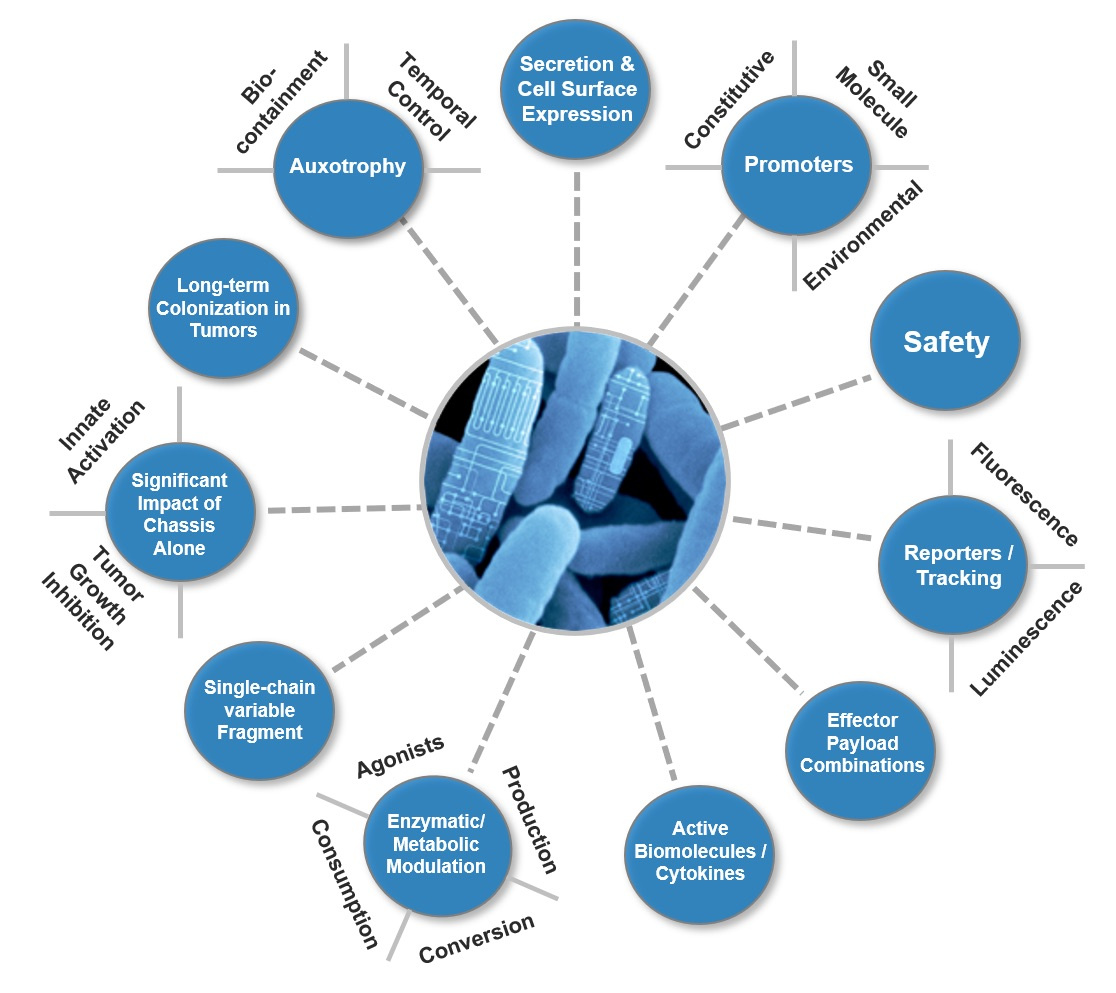
We focused on a few potential therapeutic approaches. The first is the Stimulator of Interferon Genes (STING) pathway which is promising for activating antitumor immune responses. The use of small molecule STING agonists has yielded mixed results. While the intended target is antigen-presenting cells (APCs), at high doses these agonists often lose efficacy7 as they may also be taken up by T cells triggering apoptosis and blunting the antitumor response8 .
Since bacteria can remain metabolically active and localize within tumors for days-weeks and are naturally engulfed by phagocytic APCs, we created SYNB1891 which produces a STING agonist, as a targeted therapy. Furthermore, the use of a bacterial delivery vehicle provided additional immune stimulation of its own. Keeping in mind the potential clinical application of our strain, we evaluated and incorporated critical engineering features like a control element and auxothrophies to create SYNB1891 to be efficacious, scalable, and suitable for use in humans (see Figure 2).
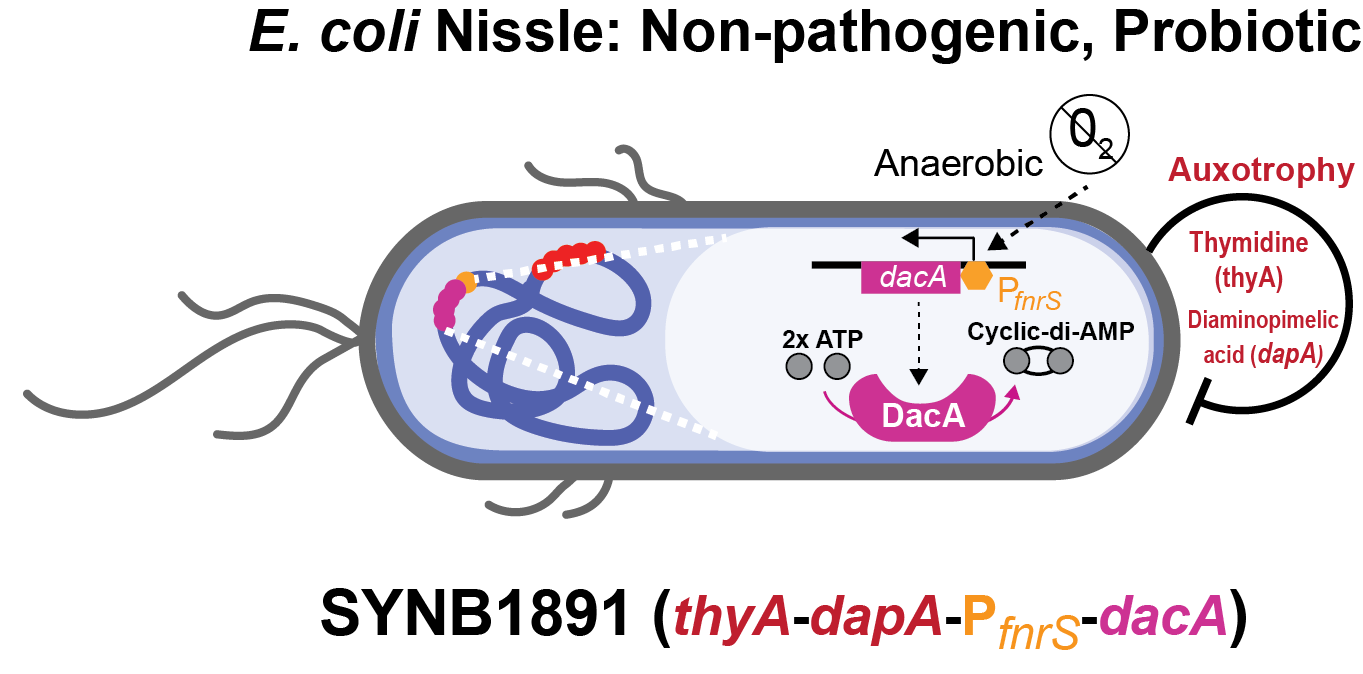
• EcN is a non-pathogenic, antibiotic, and serum sensitive bacterial chassis that is administered into the tumor. It provides initial innate immune activation and naturally targets bacterially-produced ligands and active biomolecules to phagocytic APCs.
• The DacA enzyme from Listeria monocytogenes generates the bacterial STING agonist cyclic-di-AMP.
• An anaerobically induced FNR promoter controls the expression of the dacA gene circuit. The FNR promoter shut offs at high oxygen levels during manufacturing and is later turned on upon entering the anaerobic tumor microenvironment.
• Additional safety features include dual dapA and thyA auxotrophies to inhibit bacterial expansion in a patient.
In our study we demonstrated encouraging results using SYNB1891 to treat preclinical mouse models of cancer and to activate human APCs in vitro. With strong preclinical data in hand, the next step is translation of these findings to the treatment of human cancer.
SYNB1891 is being evaluated in an ongoing Phase I clinical trial in patients with advanced solid tumors or lymphoma (NCT04167137). Positive signs of SYNB1891 efficacy and tolerability will provide further support for the potential development of additional engineered EcN strains using one or multiple sets of immunomodulatory effectors tailored to the needs of a specific cancer or patient subtypes.
Daniel Leventhal, Anna Sokolovska, Synlogic, Inc.
Full article: Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity
We thank a great team of people who had a vision, worked together to design and move this new living medicine to a clinical trial in just 2 years; and are still working today to bring SYNB1891 to patients.
1. McCarthy, E. F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 26, 154–158 (2006).
2. Felgner, S., Kocijancic, D., Frahm, M. & Weiss, S. Bacteria in cancer therapy: renaissance of an old concept. Int J Microbiol 2016, 8451728 (2016).
3. Sonnenborn, U. & Schulze, J. The non-pathogenicEscherichia coli strain Nissle 1917 – features of a versatile probiotic. Microb. Ecol. Health Dis. 21, 122–158 (2009).
4. Charbonneau, M. R., Isabella, V. M., Li, N. & Kurtz, C. B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738 (2020).
5. Isabella, V. M. et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol. 36, 857–864 (2018).
6. Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 11, (2019).
7. Sivick, K. E. et al. Magnitude of Therapeutic STING Activation Determines CD8+ T Cell-Mediated Anti-tumor Immunity. Cell Rep. 25, 3074–3085.e5 (2018).
8. Larkin, B. et al. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. J. Immunol. 199, 397–402 (2017).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in