Designing Nature-Inspired Honeycomb Aerogels for Cleaner Air and a Cooler Planet
Published in Earth & Environment and Materials
Our research showcases a new class of ultralight, porous materials—MEEG-CS aerogels—that combine graphene, metal oxides, and biopolymers to mimic nature’s most efficient structures.
When we began this work, the challenge was both simple and daunting: how do you capture a gas as elusive and omnipresent as carbon dioxide (CO₂) – and do it sustainably, efficiently, and at scale?
As climate change accelerates, so does the urgency to remove CO₂ from industrial emissions and ambient air. While current CO₂ capture technologies exist, they often come with high costs, complex regeneration steps, and issues with water stability or selectivity. We wanted to develop something simpler, cheaper, and smarter.
What if we could learn from nature’s own designs?
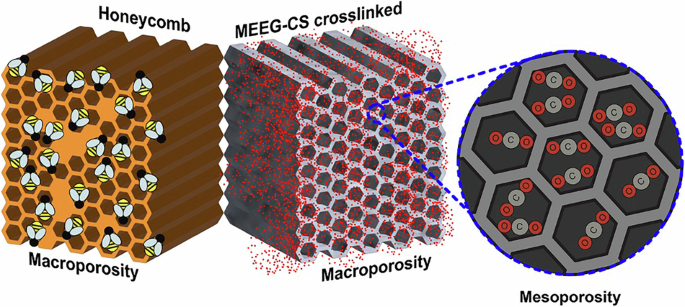
We took inspiration from bees’ honeycombs, coral skeletons, and diatom shells—structures that have evolved to be light yet strong, porous yet protective. These natural architectures provided the blueprint for our aerogel microstructure, which we replicated at both the macro- and micro-scale using advanced materials engineering.
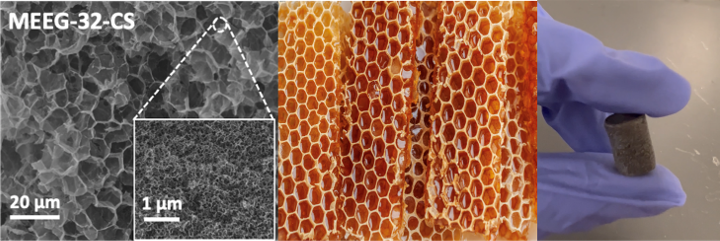
Figure: Comparison of MEEG-32-CS microstructure and beeswax hierarchical honeycomb structures observed in nature; An image of the synthesized MEEG-32-CS aerogel.
Our final material, MEEG-CS, is a hybrid aerogel made from:
- MEEG (MnO₂-functionalized electrochemically exfoliated graphene), which prevents graphene sheets from sticking together and boosts surface area
- Chitosan, a biodegradable polymer derived from shrimp shells
- A crosslinking agent (EDC-NHS), to bind the components and preserve structure during freeze-drying
The result? An ultra-light solid (think: marshmallow meets sponge) with a dual-scale honeycomb structure that traps CO₂ with exceptional efficiency.
One of the most exciting findings was that surface area alone isn’t enough. Even though many adsorbents boast very high surface areas, they often fall short in real-world conditions due to poor gas selectivity or pressure drop issues.
Our material balanced all three critical factors:
- High CO₂ uptake: 3.94 mmol/g at 298 K, outperforming many commercial and academic benchmarks
- Excellent selectivity: 65.2 times more CO₂ than N₂ under flue gas conditions
- Structural integrity in humidity: thanks to chitosan’s partial hydrophobicity and smart crosslinking
And all of this came from a material with just half the surface area of some popular silica-based adsorbents.
The Tech Behind the Magic
We used several cutting-edge techniques, including:
- Electrochemical exfoliation of graphite to produce conductive graphene sheets
- Freeze-drying and thermal treatment to preserve the delicate honeycomb architecture
- Direct Joule heating to regenerate the aerogels—made possible by the material’s inherent electrical conductivity
This last point is especially exciting: most CO₂ sorbents require bulky ovens or external heating systems. Ours can regenerate in under 20 seconds using low-voltage electricity. It’s faster, more efficient, and more scalable for industrial applications.
Behind the Scenes
One memory that stands out is how we struggled for weeks to prevent the aerogels from collapsing during the drying process. It was only after switching to EDC-NHS chemistry and adjusting the pH that the honeycomb structure finally stayed intact.
Another learning moment was our realization that MnO₂ doesn’t just enhance surface area—it also prevents the dreaded π–π stacking of graphene layers, acting like tiny spacers that keep the sheets from collapsing. That tiny tweak transformed the material’s performance.
We also had many surprises along the way. We didn’t expect the aerogels to be so stable over time. After 20 full CO₂ adsorption-regeneration cycles, our material retained over 98.9% of its capacity, and its structure showed no visible damage under SEM.
Another surprise was the low water uptake—just 7.8 mmol/g at 100% relative humidity. That’s remarkably low compared to other CO₂ adsorbents, which means the material could potentially be used in humid flue gas environments without compromising its performance.
We hope our work encourages more collaboration between material science, climate engineering, and biomimetic design. If you're working in energy, sustainability, or nanomaterials, we would love to connect and hear your thoughts.
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Multifunctional hydrogels
Publishing Model: Open Access
Deadline: Feb 28, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in