Destabilization by trimerization
Published in Bioengineering & Biotechnology, Microbiology, and Cell & Molecular Biology

Peculiarities of respiratory syncytial virus fusion protein
- High instability
Although all class I fusion proteins are inherently metastable, the fusion protein F of respiratory syncytial virus (RSV) is exceptionally unstable which was the reason that only recently, after seven decades of work, efficacious vaccines have finally been approved. It was only upon recognizing the highly unstable nature of the prefusion F protein and gaining detailed knowledge of its architecture, that the RSV vaccine field was transformed by application of prefusion-stabilizing substitutions1.
- Double cleavage
Apart from the exceptional instability, another unusual feature of RSV F is the unique maturation process, since it involves two separate cleavage events by furin-like proteases, resulting in F22, the intervening p27 peptide, and the membrane-anchored F1 fusion domain. In 2015 we discovered that the highly glycosylated p27 peptide sterically blocks trimerization and that the mature prefusion F trimer can only be formed upon release of p273. Thus, the p27 peptide functions to retain the monomer prefusion conformation during biosynthesis and likely to prevent premature trimerization and fusion activation (Figure 1).
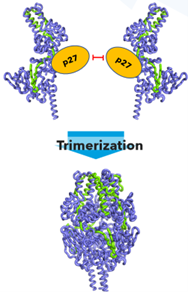
Destabilizing effect of trimerization
Typically, multimerization increases stability of a protein assembly and destabilization by multimerization may seem counter intuitive. In the case of the RSV prefusion F trimer, the extensive interprotomer contacts make a compact structure and seemingly the static X-ray structure of the tightly intertwined trimer looks stable1. Surprisingly, in our study we show that it is the monomer that retains a stable prefusion conformation, but upon trimerization, the trimer rapidly destabilizes and transforms into the postfusion conformation.
Therefore, the p27-mediated delay of RSV preF trimerization is linked to the high trimer instability, and linkage to a heterologous trimerization domain like foldon leads to rapid refolding to the postfusion F conformation.
While high instability upon trimerization surprised us, it actually makes sense from a mechanistic perspective since an unstable high-energy fusion protein is needed to drive fusion.
Dropping the foldon
Interestingly, all current licensed RSV subunit vaccines address the stabilization and trimerization challenge by incorporating C-terminal foldon trimerization domains, not realizing that the use of foldon needs to be compensated by additional stabilizing substitutions. We show that current RSV vaccines induce undesirable anti-foldon antibodies in non-human primates (NHP), mice and humans. Apart from the possibility of side-effects, the off-target response could impact vaccine potency after repeated administrations or the potency of future vaccines that contain a foldon domain and even possible future therapeutics that contain a foldon domain. Our investigation in the RSV F prefusion instability and the solutions brought forward had an appealing application for development of a possible foldon-free RSV vaccine. Recognizing the drawbacks of the destabilizing effect of the foldon domain and the off-target response, we asked ourselves the question whether it would be possible to make an efficacious vaccine using a prefusion RSV F trimer without having to use foldon. Therefore, we set out to elucidate the structural basis of trimerization-induced preF destabilization.
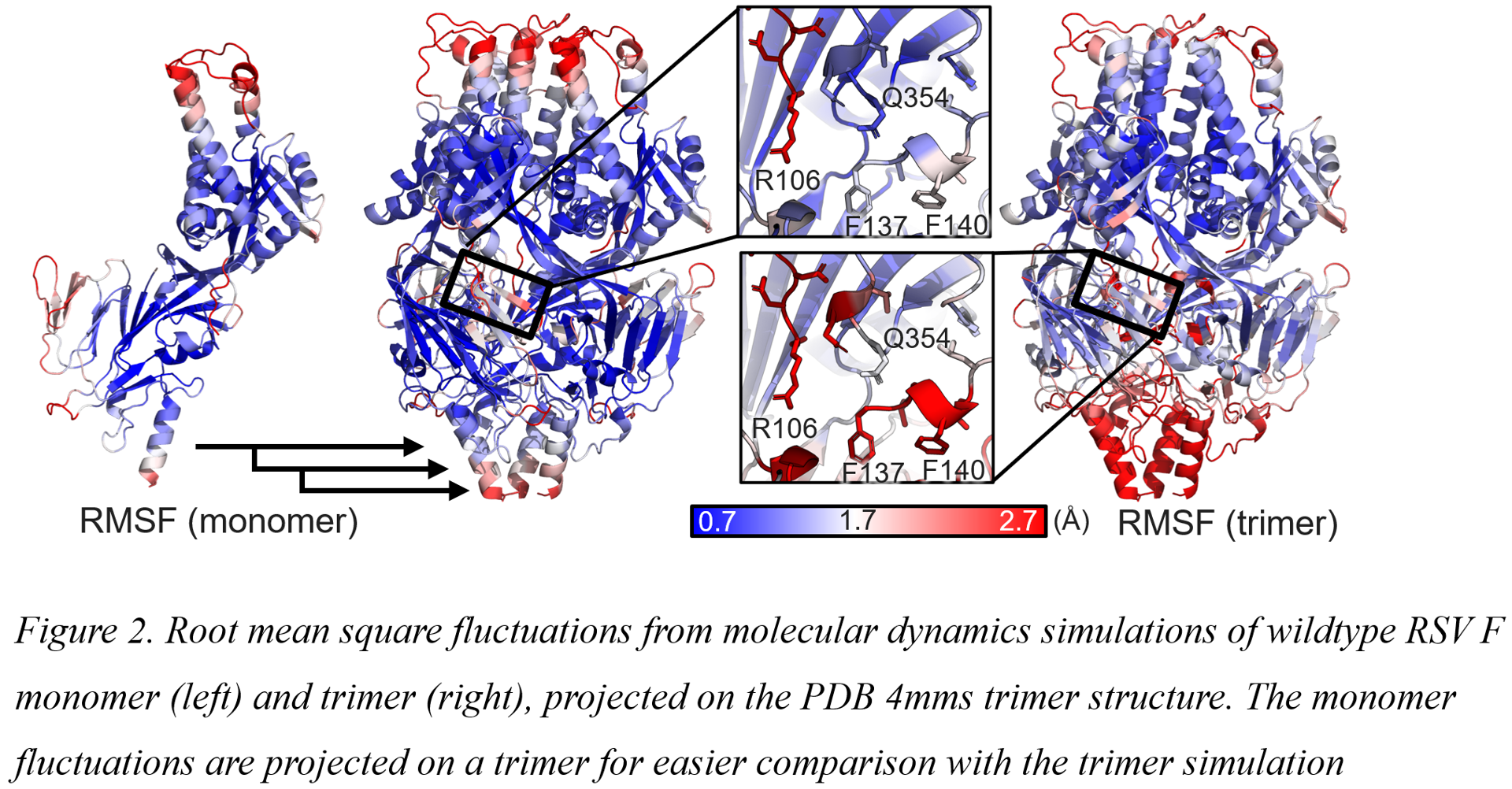
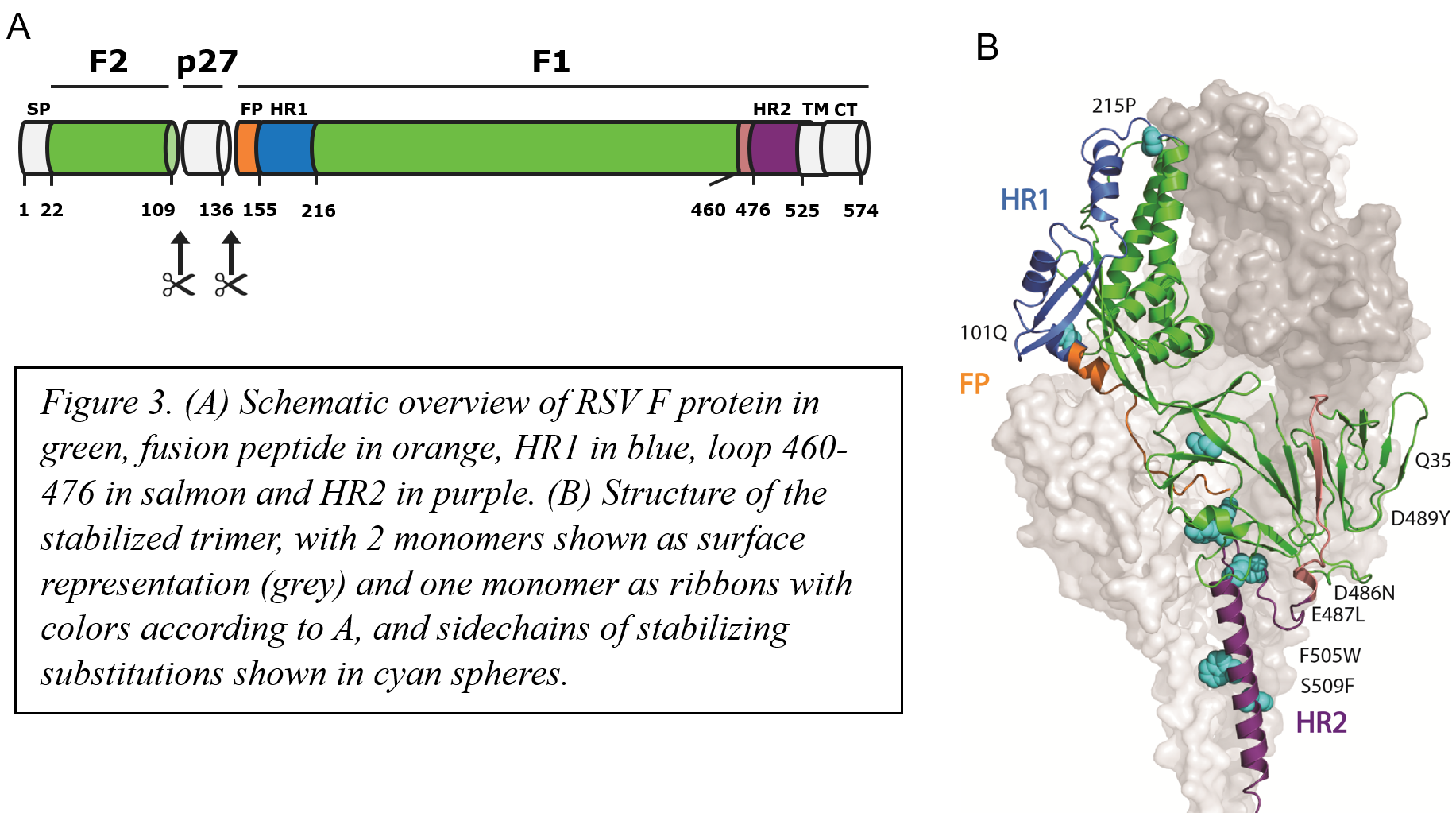
By using molecular dynamics (MD) simulations to simulate breathing movement of an RSV F monomer versus an F trimer, we were able to pinpoint multiple charged regions of instability that form upon trimerization (Figure 2). These hotspots of instability all cluster near the base of the F trimer. Around the 3-fold axis at the nexus of the stem and the head, just below the fusion peptide, a ring of negative charges is formed upon trimerization and in the plane of the fusion peptide positive charge repulsion is formed upon trimerization that involves the positively charged N-terminus of the fusion peptide. MD showed how the negatively charged cluster is unstable and highly mobile with D486 switching between stabilizing and destabilizing positions. The tip of the fusion peptide also shows increased mobility upon trimerization and within the central cavity, the 354-357 loop shows mobility and as a result, Q354 is not able to form a stable interaction with the fusion peptide. By focusing our design efforts on these hotspots, we were able to engineer substitutions that negate these charge imbalances and improved the interface of the stem which resulted in a preF trimer that was no longer dependent on the foldon trimerization domain. The resulting preF protein is highly stable and has been structurally validated by antigenic and Cryo-EM analysis (Figure 3).
The preF is immunogenic and protective in naïve mouse models and boosts neutralizing antibody titers in RSV pre-exposed mice and non-human primates, while achieving similar titers to approved RSV vaccines in mice. This stable preF design is a promising option as a foldon-independent candidate for a next-generation RSV vaccine immunogen.
References
- McLellan, J. S. et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342, 592–598 (2013).
- González-Reyes, L. et al. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc Natl Acad Sci U S A 98, 9859–9864 (2001).
- Krarup, A. et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun 6, 8143 (2015).
MJG Bakkers, JPM Langedijk, ForgeBio
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in