Directional long-distance electron transfer from reduced to oxidized zones in the subsurface
Published in Earth & Environment
Electron transfer (ET) is the fundamental process for life on Earth, and ET over different distances is central to most biogeochemical processes in the subsurface. Short-distance ET at nanometers to micrometers has been a research hotspot for several decades. Whereas, along the subsurface redox profiles, the driving force of ET, is directional and long-distance in the scale of centimeters or even meters. Thus, an intriguing question arises regarding whether and how ET can reach such a long distance along the redox gradient. The redox gradients along with microbes and redox-active species are ubiquitous in natural and engineered subsurface systems, wetlands, and waste and wastewater biotreatments. To answer this scientific question, here we hypothesize that a ubiquitous long-distance ET chain exists along the redox gradient in the subsurface, which is composed of the sequential series of many short-distance primary ET processes from low to high reduction potentials (Figure 1).
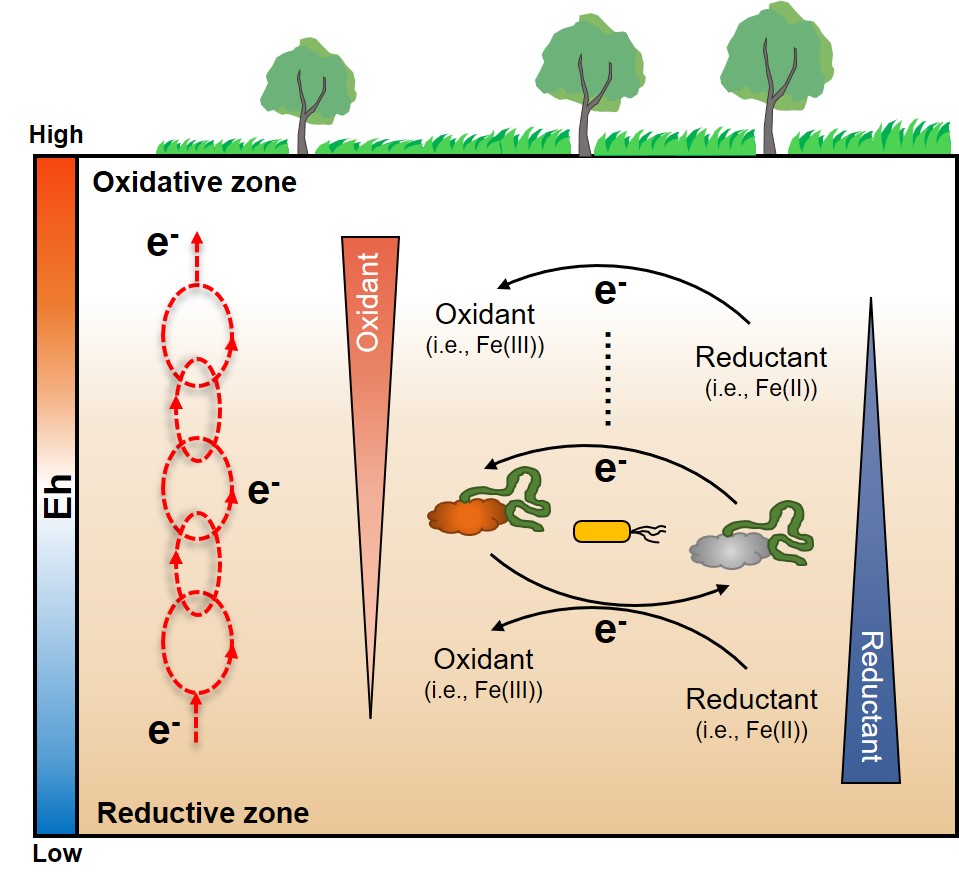
Figure 1. Conceptual model of directional long-distance ET chain along subsurface redox gradient. The red circles in the left conceptualize the series of short-distance ET between connected redox couples from low to high reduction potentials. The redox couples in the right present the biological and chemical mechanisms for the ET.
To evaluate our hypothesis, sediment column experiments were carried out to simulate the subsurface redox profiles. In a typical column (length: 10.6 cm), we filled the bottom half with reduced sediments, and the upper half with the same quantity of oxidized sediments (Figure 2a). Three types of sediments were chosen to fill separately in each column. By using electron-donating capacity (EDC) variation as a proxy of ET, we measured the temporal variation in EDC values (DEDC) to reflect ET process along the redox gradient in the sediment columns.
For the clay loam sediment, DEDC values within 24 d at 1.3 cm above the oxidized/reduced interface increased gradually to 14.8 μmol e-/g; at -1.3 cm below the interface decreased to -14.2 μmol e-/g; and their sum at around 0 reveals a nearly symmetric variation in the oxidized and reduced halves (Figure 2b). Nearly symmetric DEDC variations at different locations of the oxidized and reduced halves were also observed at different time, with the variation decreased from the zones near interface (1.3 and -1.3 cm) to the two ends (5.3 and -5.3 cm) (Figure 2c). Similar observations were obtained for silt and sand loam sediments, with the symmetric DEDC variation less pronounced for sand loam sediment (Figure 2d, e). These results show a long-distance ET chain at the scale of ~10 cm in the column. Based on redox species (Fe and organic matter) and microbial activity analyses in the sediments, this ET chain proceeds synergistically through a long-distance ET chain constituted by a series of short-distance electron hopping reactions associated with microbes and redox-active species like iron and organic matter.
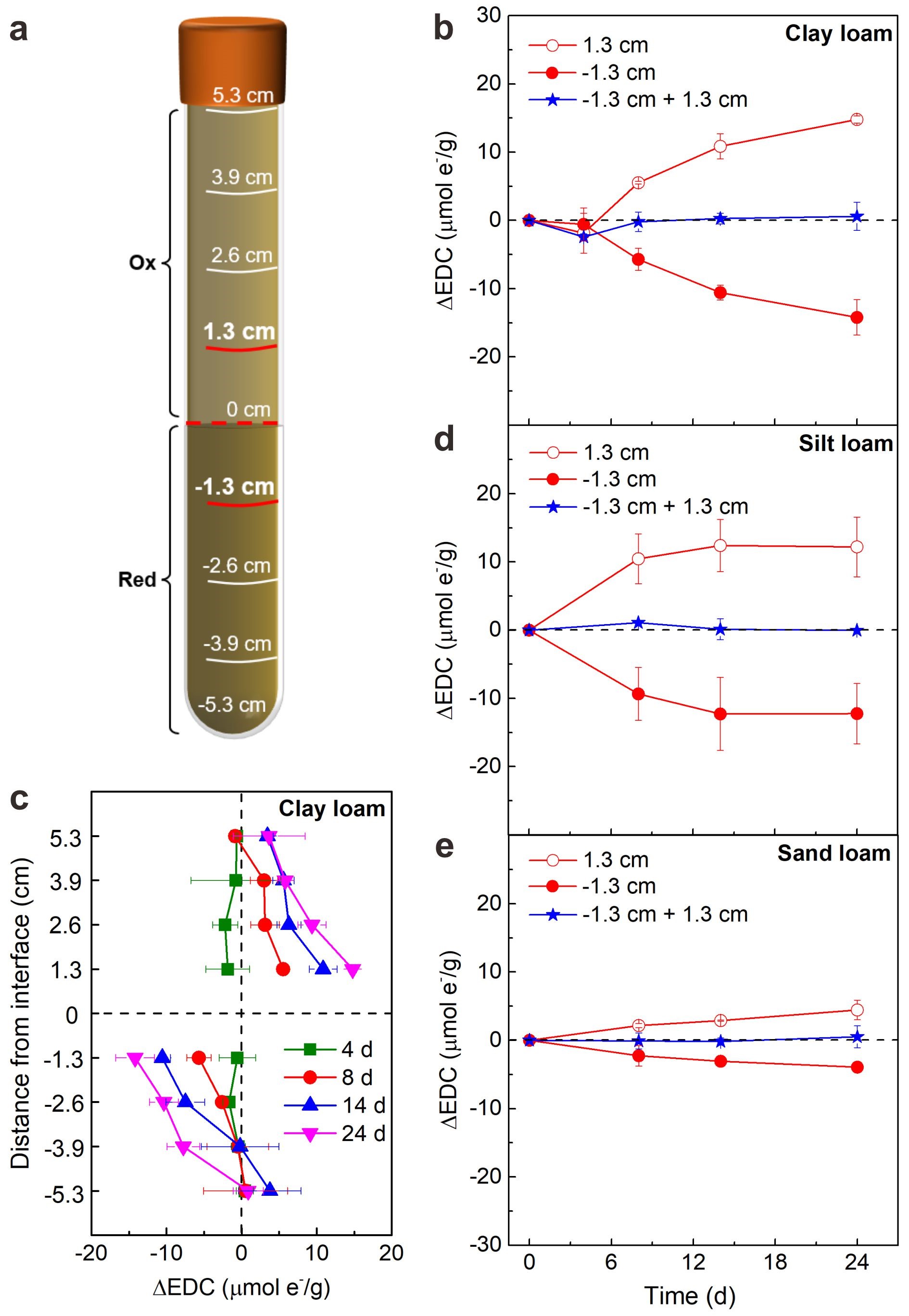
Figure 2. Long-distance ET in different textures of anoxic sediment columns. (a) Schematic of column filled with oxidized (“Ox”) and reduced (“Red”) sediments in the upper and bottom halves, respectively. (b) Temporal variation of DEDC near the oxidized/reduced interface (locations at 1.3 and -1.3 cm) and (c) spatial variation of DEDC in the clay loam columns at different time. Temporal variation of DEDC near the oxidized/reduced interface (locations at 1.3 and -1.3 cm) in the (d) silt loam and (e) sand loam columns. DEDC was calculated by the value difference between sampling and initial time. The DEDC values for “-1.3 cm + 1.3 cm” were calculated by the sum of values at locations at 1.3 and -1.3 cm in different textures of sediment columns.
In order to quantitatively the long-distance ET, we further calculated the ET flux across the oxidized/reduced interface in the sediment columns by summing DEDC in the whole reduced half. Under the initial redox gradient of ~700 mV, the ET flux was estimated to be 6.73 μmol e-/(cm2·d) for the reduced half. The ET flux indicates that 6.73 μmol e- per day can be transported from deep reduced sediments across a 1-cm2 area to upper oxidized sediments. Both microbial and chemical processes synergistically mediate the long-distance ET chain. This directional long-distance ET represents an overlooked but important “remote” source of electrons for the local short-distance ET, influencing the local biogeochemical and environmental processes.
The expected significance of directional long-distance ET can be even more exciting than the conclusions mentioned above. Taking the shallow subsurface (i.e., soils, sediments, and wetlands) as a typical example, people previously estimated the flux of CO2, N2O and CH4 emission only by the quantity of electrons (carried by electron donors in the forms of ferrous iron or organic matter) in the shallow layers, without considering the contribution of electrons from deeper layers along redox gradients. The proposed long-distance ET suggests that the estimation is insufficient. The contribution of electrons from deep layers can be important for the subsurface with favorable conditions for long-distance ET, and the importance increases with the time with the gradual consumption of electrons in the surface layers. Moreover, as redox gradient and ET mediators are ubiquitous in many scenarios, the long-distance ET is expected to be important therein. For example, redox gradients in the subsurface can be created by water table fluctuations, soil and groundwater remediation activities, underground construction activities, water conservancy projects (i.e., damming), and so on. Redox gradient also occurs in waste composting treatment, activated sludges and anaerobic sludges for wastewater treatment.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in