In the last decade, several studies have converged on the nature of the microenvironment of normal hematopoietic stem cells (HSC) and leukaemia stem cells (LSCs), namely the bone marrow microenvironment (BMM). The BMM is a complex entity consisting of various cell types, the extracellular matrix, soluble and other factors. This BMM influences the (patho-) physiology of normal HSC and LSC, and may be a possible therapeutic target in leukaemia1,2. LSCs are highly dependent on this BMM, as interactions of leukaemia cells with the different cellular and acellular components of the BMM mediate pro‑survival mechanisms and protect LSCs from conventional and targeted therapies.
Characterisation of the components of the BMM and identification of the interactions and dependencies between leukaemia cells and their environment are necessary for possible targeting strategies. Modulation of specific interactions between leukaemia cells and their BMM or modulation of the BMM itself, rendering it inhospitable for malignant cells and ultimately eradicating leukaemia stem cells, are novel and promising strategies to augment existing therapies in leukaemia 3.
As of yet, relatively little attention has been directed towards the role of chemical factors in the leukaemic BMM, such as calcium ions, which are important constituents of the BMM. In fact, calcium is the most abundant mineral in the body, a key component of bones. It is released by (parathyroid hormone (PTH))‑induced) bone remodelling. Calcium ions play a role in the localisation, engraftment and adhesion of normal HSC to extracellular matrix (ECM) proteins in the BMM via the calcium sensing receptor (CaSR), a G-protein coupled receptor, thereby maintaining normal haematopoiesis 4,5. In addition, CaSR has been implicated in the development of different cancers, functioning as either tumour suppressor or oncogene, depending on the involved tissue 6. However, the role of CaSR and its associated pathways in the local BMM for the development of leukaemia was not understood.
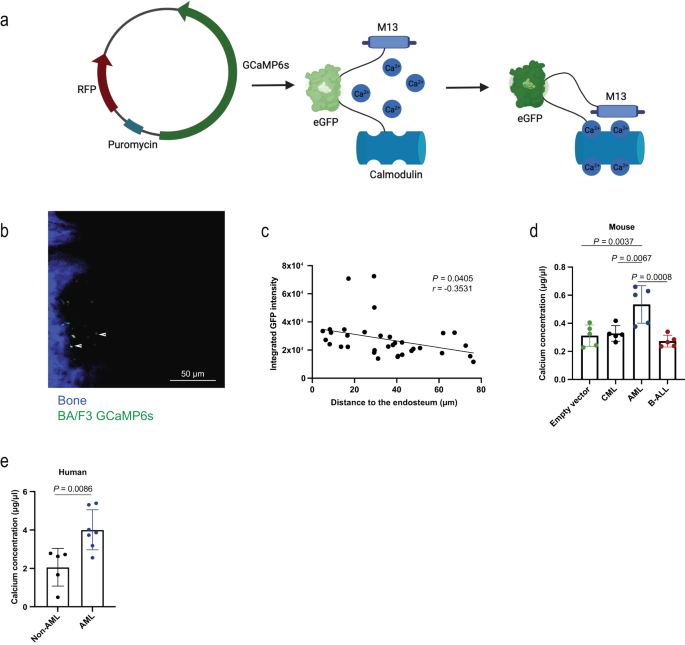
In our article Pereira et al., Nat Comm, 2023, we showed that calcium ions are not uniformly distributed within the BMM, but instead form a gradient with highest calcium concentrations close to the endosteum. In fact, calcium ions have very specific effects on leukaemia cells, as CaSR regulates the location of MLL‑AF9+acute myeloid leukaemia (AML), but not chronic myeloid leukaemia (CML) cells within the BM.
Further, the calcium content in the BMM is higher in AML than in other leukaemias, and calcium exposure has a significant influence on adhesion, migration, intracellular calcium and CaSR and CXCR4 (C-X-C chemokine receptor type 4) expression on AML cells, while having no effect on CML cells. Additionally, CaSR is differentially expressed on distinct types of leukaemia with highest expression on AML cells. In addition, it plays highly differential roles for the development of CML, BCR‑ABL1-driven B‑ALL (B‑cell acute lymphoblastic leukaemia), and MLL‑AF9- or MN1-driven AML. Specifically, deficiency of CaSR on B‑ALL- and CML-initiating cells leads to disease acceleration, while this leads to disease prolongation in AML. Conversely, overexpression of CaSR on CML-initiating cells leads to disease prolongation, while this leads to disease acceleration in AML. Furthermore, secondary and limiting dilution transplantation of CaSR-deficient AML-initiating cells, assays to assess stem cell number and function, revealed that CaSR is necessary for stemness and self-renewal capabilities of LSCs in AML.
Focusing on MLL‑AF9-driven AML, CaSR was shown to regulate proliferation, cell cycle, apoptosis, generation of reactive oxygen species (ROS), differentiation, and DNA damage. Molecularly, CaSR interacts with filamin A, a crosslinker of actin filaments, and modulates MAPK-ERK and Wnt β-catenin signalling and intracellular levels of calcium in AML cells. Overexpression of CaSR leads to a higher pERK/ERK ratio and higher β-catenin, MYC and cyclin D1 levels, contrasting with our observed downregulation of these proteins when CaSR was depleted.
In vivo inhibition of CaSR with the specific antagonist NPS-2143 in combination with standard chemotherapy reduced tumour burden and prolonged survival of mice with AML in syngeneic and xenogeneic models more efficiently than chemotherapy alone. This suggests that combination treatment of conventional chemotherapy plus CaSR-inhibition may improve clinical outcome in AML patients.
Taken together, these findings suggest that CaSR and possibly calcium ions from the BMM differentially impact leukaemia cells and influence leukaemia progression. We propose that targeting of CaSR signalling may represent a novel candidate in the arsenal of leukaemia treatments, which may be combined with conventional therapeutic strategies.
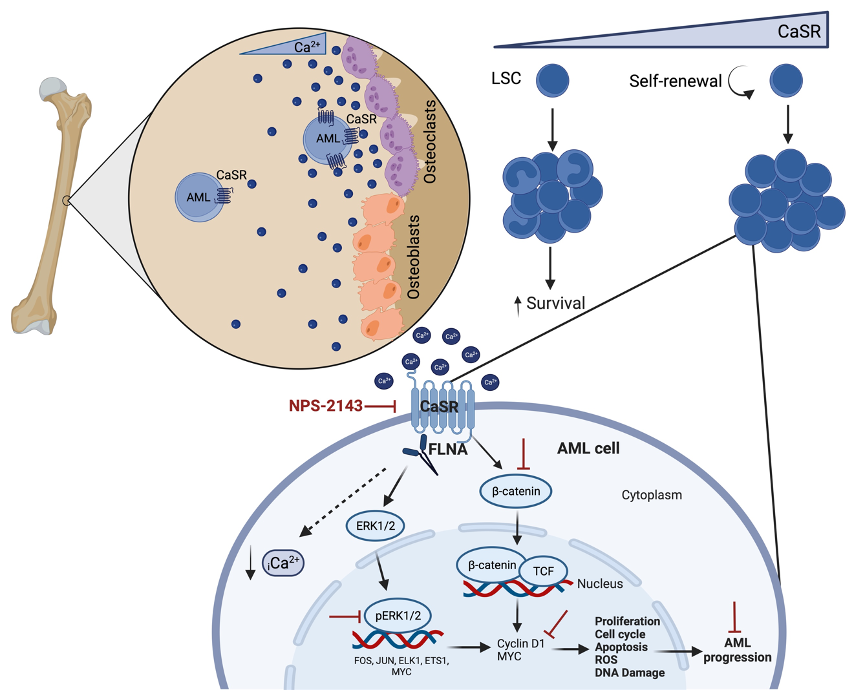 Figure 1. Schematic representation of the proposed mechanism for CaSR signalling in AML.
Figure 1. Schematic representation of the proposed mechanism for CaSR signalling in AML.
References
1 Krause, D. S. et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med 19, 1513-1517 (2013). https://doi.org:10.1038/nm.3364
2 Méndez-Ferrer, S. et al. Bone marrow niches in haematological malignancies. Nature Reviews Cancer 20, 285-298 (2020). https://doi.org:10.1038/s41568-020-0245-2
3 Krause, D. S. & Scadden, D. T. A hostel for the hostile: the bone marrow niche in hematologic neoplasms. Haematologica 100, 1376-1387 (2015). https://doi.org:10.3324/haematol.2014.113852
4 Adams, G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599-603 (2006). https://doi.org:10.1038/nature04247
5 Lam, B. S., Cunningham, C. & Adams, G. B. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood 117, 1167-1175 (2011). https://doi.org:10.1182/blood-2010-05-286294
6 Tennakoon, S., Aggarwal, A. & Kállay, E. The calcium-sensing receptor and the hallmarks of cancer. Biochim Biophys Acta 1863, 1398-1407 (2016). https://doi.org:10.1016/j.bbamcr.2015.11.017
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in