Do long-term spaceflights alter our biological clock and influence aging?
Published in Healthcare & Nursing
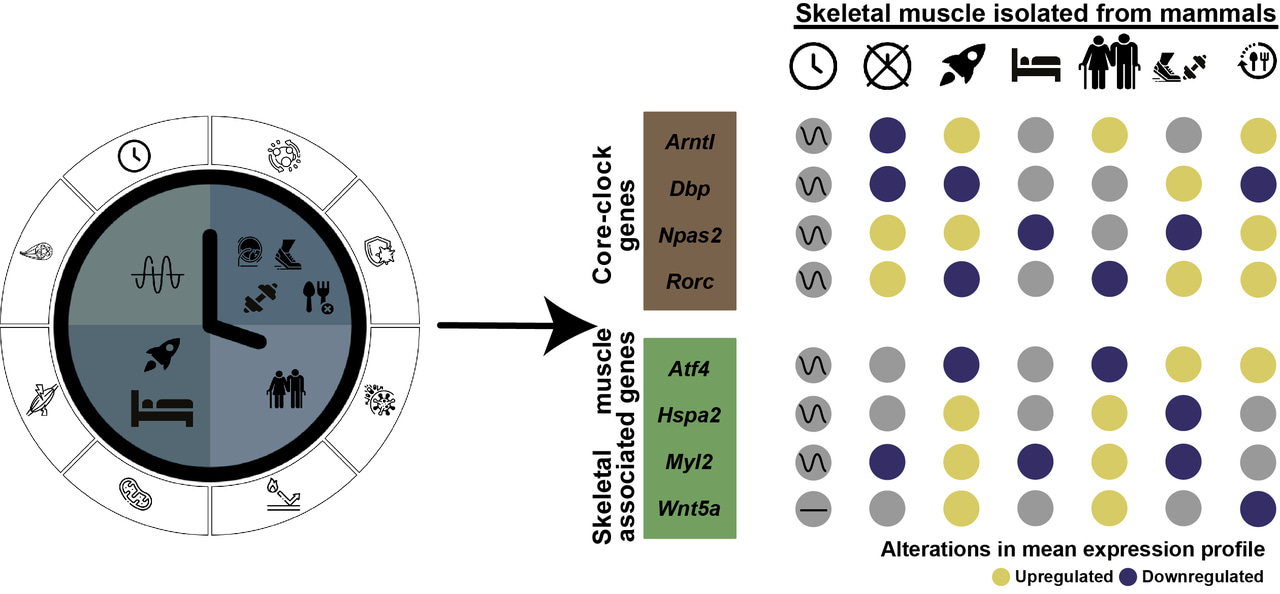
Humans have an internal biological clock, which dictates time and assures an optimal adaptation of physiology and behavior to the 24-hours Earth’s rotation. This clock also named -circadian clock- generates ~24 h (circadian) rhythms in every body cell. Circadian rhythms in gene and protein expression are generated in every body cell via well-coordinated transcriptional-translational feedback loops1. To assure optimal body homeostasis, a central pacemaker in the suprachiasmatic nucleus, in the brain (hypothalamus), receives entrainment inputs from the external environment (such as light) and can thus maintain all other (peripheral) clocks (e.g. skeletal muscle2 and liver) within the body in synchrony also with the geophysical time3.
In mammals, the dysregulation of the circadian clock is an important characteristic of age-related alterations in for example myogenesis and metabolism4 and is associated with musculoskeletal atrophy during crewed spaceflight5. However, detailed insights into the molecular alterations in the circadian clock due to spaceflight and aging in skeletal muscle remain unknown. The risk of muscle wasting due to spaceflights among astronauts increases with the duration of Space travel. Also on Earth, the progressive muscle loss due to aging (sarcopenia) presents a major socioeconomic burden on our society.
In our study, we carried out a comprehensive bioinformatics analysis to investigate the consequences of clock dysregulation during spaceflights and searched for similar characteristics to age-related changes observed on Earth, in mammalian skeletal muscle. Using omics datasets from mammals (mice and humans), we identified gene expression alterations in both the circadian clock and skeletal muscle-associated signalling pathways (e.g. mTOR and MAPK) as a result of spaceflights (in particular long-term spaceflights) that resemble age-related changes observed on Earth. The core-clock gene Arntl (also known as Bmal1) and the skeletal muscle-related gene Hspa2 showed differential upregulation due to long-term spaceflight and aging on Earth. Furthermore, our results also show that external interventions, like physical exercise or fasting (for example, time-out from energy intake across the day) leads to molecular changes in the circadian clock that may compensate for the disruption of the clock observed during spaceflight.
Thus, maintaining clock function is critical to ameliorate the non-physiological changes and musculoskeletal atrophy observed in otherwise healthy astronauts and among the elderly on Earth. With the further development of space medicine, future studies will be needed to confirm and explore such results. The accumulated evidence so far indeed motivates the need of considering circadian rhythm variations and of developing ways to overcome them. Circadian rhythms are thus likely to play a relevant role in future space explorations and considerations of day length on other planets will be relevant factors to consider to allow for an entrainment of our endogenous clocks to day lengths which are very different from those on Earth.
References
1. Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18, 164-179 (2017). https://doi.org:10.1038/nrg.2016.150
2. Harfmann, B. D., Schroder, E. A. & Esser, K. A. Circadian rhythms, the molecular clock, and skeletal muscle. Journal of biological rhythms 30, 84-94 (2015). https://doi.org:10.1177/0748730414561638
3. Bollinger, T. & Schibler, U. Circadian rhythms - from genes to physiology and disease. Swiss medical weekly 144, w13984 (2014). https://doi.org:10.4414/smw.2014.13984
4. Yamazaki, S. et al. Effects of aging on central and peripheral mammalian clocks. Proceedings of the National Academy of Sciences of the United States of America 99, 10801-10806 (2002). https://doi.org:10.1073/pnas.152318499
5. Stein, T. P. Nutrition and muscle loss in humans during spaceflight. Advances in space biology and medicine 7, 49-97 (1999). https://doi.org:10.1016/s1569-2574(08)60007-6
Follow the Topic
-
npj Microgravity

This journal aims to provide a thorough understanding of the scientific impact and future of spaceflight research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Human System Risk Management and Knowledge Graphs for Human Spaceflight - Volume II
Publishing Model: Open Access
Deadline: Oct 31, 2026
Space Biomanufacturing
Publishing Model: Open Access
Deadline: Jun 15, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in