Double-walled Al-based MOF with large microporous specific surface area for trace benzene adsorption
Published in Chemistry
Trace benzene vapor can harm the human body. Double-walled metal-organic frameworks (MOFs), synthesized using Zn and Co, are potential porous materials for trace benzene adsorption, as evidenced by double-walled MOFs, such as BUT-53(Co), BUT-57(Co), and BUT-58(Zn). Compared to Zn and Co metal ions, aluminum is abundant in nature and low-toxicity. The specific surface areas of reported double-walled MOFs are in the range of 849-1128m2/g, limiting their trace benzene adsorption. Therefore, designing and synthesizing double-walled Al-based MOF with high microporous surface area is a better strategy for trace benzene adsorption. Herein, we synthesize a novel Al-based MOF material, ZJU-520(Al), with a high microporous surface area of 2235m2/g, and excellent chemical-thermal stability. It has trace benzene adsorption of 5.98 mmol/g at P/P0 = 0.01, higher than reported materials for benzene adsorption. Adsorbed benzene molecules are trapped on two types of sites. One (site I) is near the AlO6 clusters, another (site II) is near the N atom of ligands. Besides, ZJU-520(Al) also can separate trace benzene from the benzene/cyclohexane mixture, due to higher adsorption affinity with benzene.
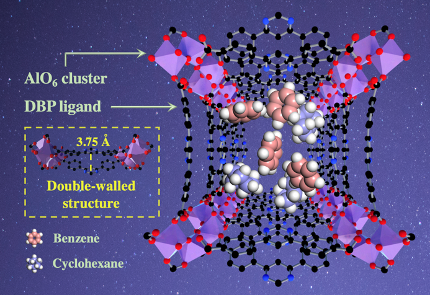
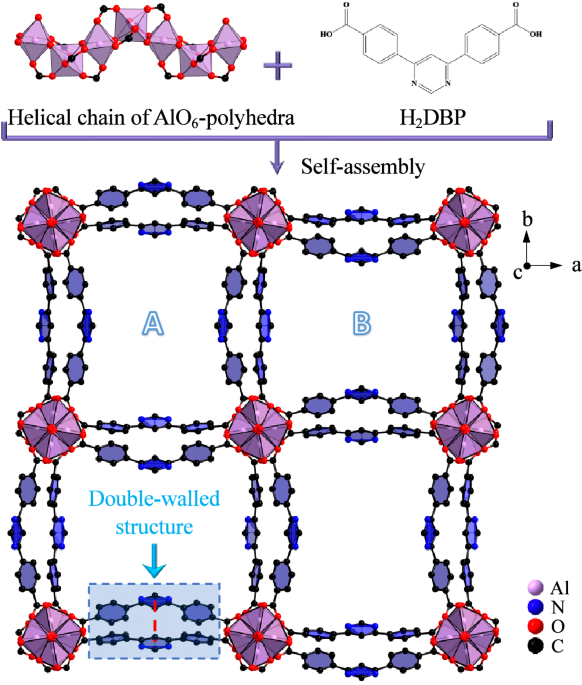
Structural characterization. The double-walled ZJU-520(Al) is consisted by helical chain of AlO6 clusters and 4,6-Di(4-carboxyphenyl)pyrimidine ligands, in the tetragonal crystal system with I41md space group and lattice parameters (a = b = 36.65 Å, c = 10.56 Å). It is with two types of 1D channels, named as A with narrow channel and B with wide channel here (Fig. 1), respectively. Those two channels are formed due to the 11.01° rotation of AlO6 clusters.
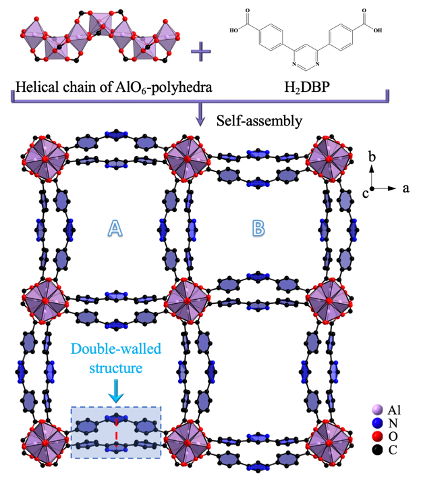
Fig. 1 Crystal structure of ZJU-520(Al). Structure description of double-walled ZJU-520(Al), with two types of micropores (named as A and B), consisted by helical chain of AlO6-polyhedra and H2DBP ligand, as viewed from c axis, and the distance of double-wall on ZJU-520(Al) in red dotted line.
Specific surface area and stability. The microporous specific surface area of ZJU-520(Al) is up to 2235 m2/g (Fig. 2a), higher than the reported double-walled MOFs (849-1128 m2/g). Its pore volume is 0.84 cm3/g, with pore size distribution in the range of 9.26-12.99Å. The interplanar spacing is 12.83 Å along the (2, 2, 0) direction, observed from high-resolution transmission electron microscopy (HRTEM) image (Fig. 2b), in agreement with the theorical interplanar spacing of 12.96 Å. ZJU-520(Al) is with thermal stability until 250 ℃, identified by the thermal gravimetric analysis (TGA) of activated ZJU-520(Al) (Fig. 2c). The framework of ZJU-520(Al) also exhibits excellent chemical stability, identified by the N2 adsorption-desorption isotherms (Fig. 2a) and PXRD patterns (Fig. 2d) of ZJU-520(Al) without obvious changes, after being treated with water (pH = 7), HCl solution (pH = 4) and NaOH solution (pH = 12).
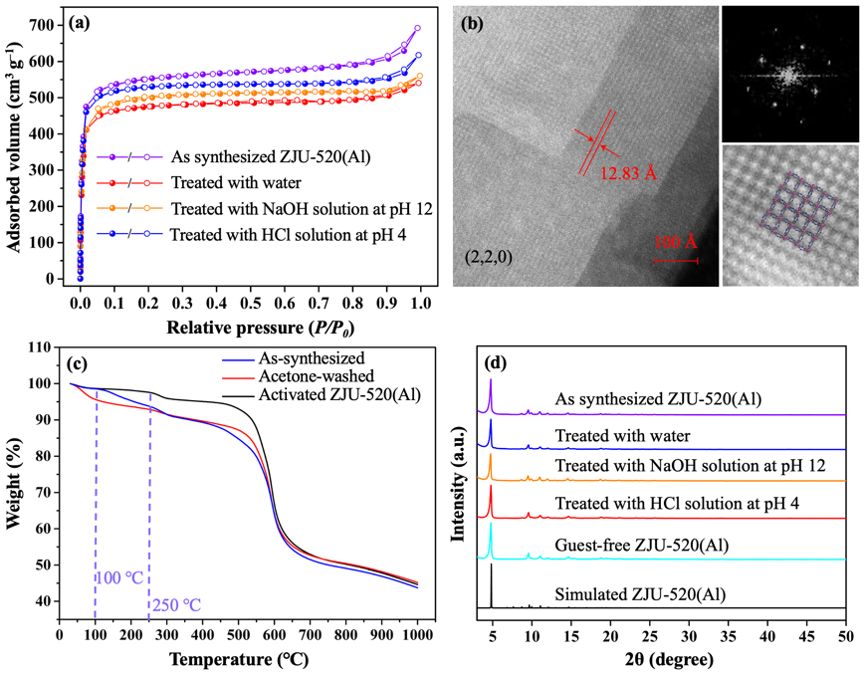
Fig. 2 N2 adsorption-desorption isotherms in filled-white circles, respectively (a), HRTEM image taken along the (2, 2, 0) direction (b), TGA curves (c) and PXRD patterns (d) of ZJU-520(Al) with and without treatment.
Static and dynamic adsorption. ZJU-520(Al) can adsorb trace benzene up to 5.98 mmol/g (Q0.01) at 298 K and P/P0 = 0.01 (Fig. 3a,b and source data), higher than double-walled BUT-54(Co) (Q0.01 = 4.31 mmol/g) and other previously reported benzene adsorbents (Fig. 4a), including PAF-1 (Q0.01 = 3.65 mmol/g), ZJU-620(Al) (Q0.01 = 3.80 mmol/g) and UiO-66(CuII) (Q0.01 = 3.92 mmol/g). It also has excellent saturation adsorption capacity for benzene (12.07 mmol/g), toluene (6.86 mmol/g), ethylbenzene (3.73 mmol/g), ortho-xylene (3.94 mmol/g), meta-xylene (3.65 mmol/g), para-xylene (4.20 mmol/g) and cyclohexane (5.27 mmol/g) at 298 K (Fig. 3a, b). Even at 308 K, ZJU-520(Al) can keep excellent trace benzene adsorption of 3.63 mmol/g (Q0.01) and rapidly up to 8.09 mmol/g at P/P0 = 0.02 (Fig. 4b), and with excellent regenerate ability due to benzene adsorption without obvious loss at least 4 times (Fig. 4c). The isosteric heat (∆H) values of benzene adsorption on ZJU-520(Al) are modestly high and increased in two steps (Fig. 4d), calculated from the isotherms at four temperatures (Fig. 4b). One step is increased at low benzene adsorption (0.00013 – 1.10 mmol/g) with the ∆H values of 39.68 – 41.59 kJ/mol owing to the dominance of guest – host interactions. Another step is increased at high benzene adsorption (3.56 – 9.79 mmol/g) with the ∆H values of 40.02 – 45.54 kJ/mol due to strong guest – guest interactions.
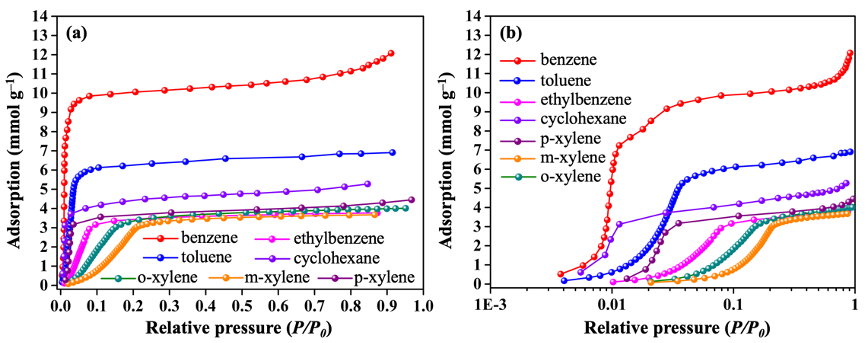
Fig. 3 Adsorption isotherms of BTEX and cyclohexane by ZJU-520(Al) at 298 K with P/P0 at normalized scale (a) and logarithmic-scale (b).
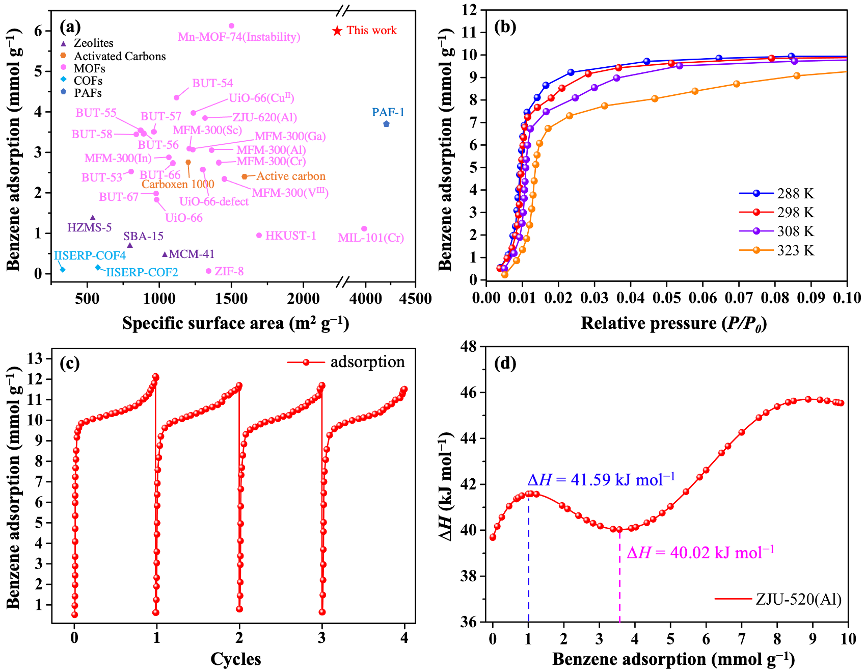
Fig. 4 Adsorption of benzene at 298 K and P/P0 = 0.01 by adsorbents with their specific surface area (a), and adsorption isotherms of benzene with P/P0 up to 0.10 at 288, 298, 308 and 328 K (b), cyclical benzene adsorption isotherms (c) as well as isosteric heat (d) of ZJU-520(Al).
Adsorption mechanism. There exist two types of benzene binding conformations, i.e., site I near the AlO6 clusters and site II near the N atom of ligands (Fig. 5a,b,d), due to host – guest interactions (Fig. 4d), such as Al – π interactions and C – H ⋯ N interactions, identified by the slices of calculated potential field. With the P/P0 up to 0.10, benzene molecules also can be adsorbed in the center of channel (Fig. 5c, e), due to strong guest – guest interactions (Fig. 4d).
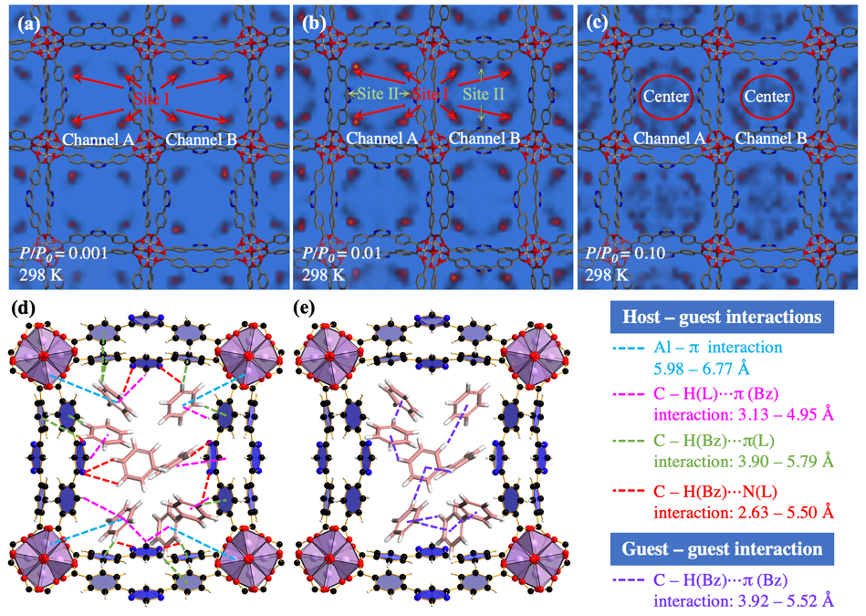
Fig. 5 Potential fields of benzene on ZJU-520(Al) at P/P0 = 0.001 (a), 0.01 (b) as well as 0.10 (c), and interactions, including host – guest interaction (d) and guest – guest interaction (e).
Separation of benzene and cyclohexane. Separating benzene (Bz) from cyclohexane (Cy) is a difficult task in industries such as nylon production, due to similar boiling points of Bz (353.25 K) with Cy (353.85 K) and similar kinetic diameters of Bz (5.90 Å) with Cy (6.20 Å) (Fig. 6a). The ideal adsorbed solution theory (IAST) selectivity value of Bz/Cy mixture at vapor volume of 50/50 by ZJU-520(Al) is 29.86, exceeding that by CUB-5 (4.2), MFOF-1 (5.3) and Mn-TCNQ-bpy (15.2). Pure cyclohexane elutes with high-purity firstly, whereas trace benzene is still adsorbed in the fixed bed and retained with a longer time course (Fig. 6b). ZJU-520(Al) is also with excellent recyclability for Bz/Cy separation at least 4 times (Fig. 6c). ZJU-520(Al) exhibits more multiple interactions with benzene than that with cyclohexane (Fig. 6d, e), resulting in the excellent Bz/Cy separation of ZJU-520(Al), even at trace benzene concentration.
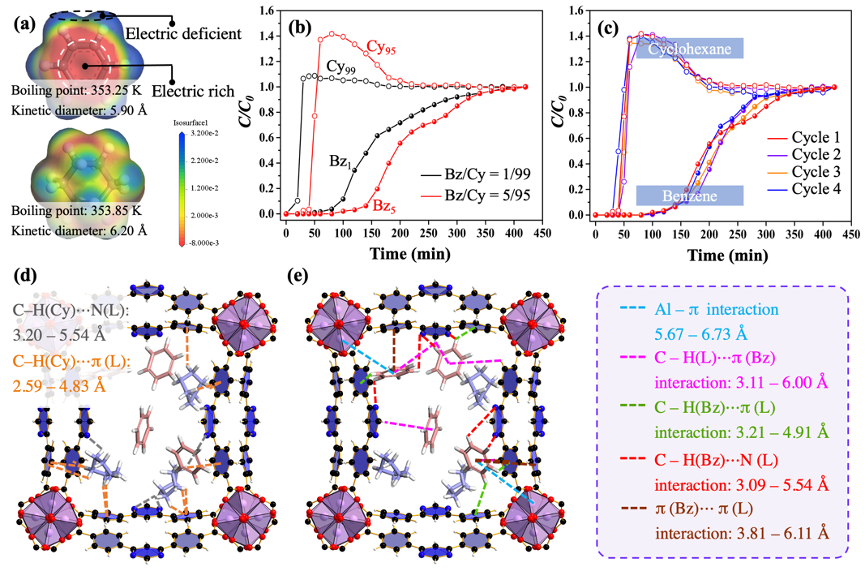
Fig. 6 Physical properties of benzene and cyclohexane (a), breakthrough curves for benzene/cyclohexane separations (b), cyclical benzene/cyclohexane separations (c) on ZJU-520(Al), and its interactions with cyclohexane (d) and benzene (e).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in