Early-Life Stress and Schizophrenia: Exploring the Role of Inhibitory Neurons in the Thalamus
Published in Neuroscience and General & Internal Medicine
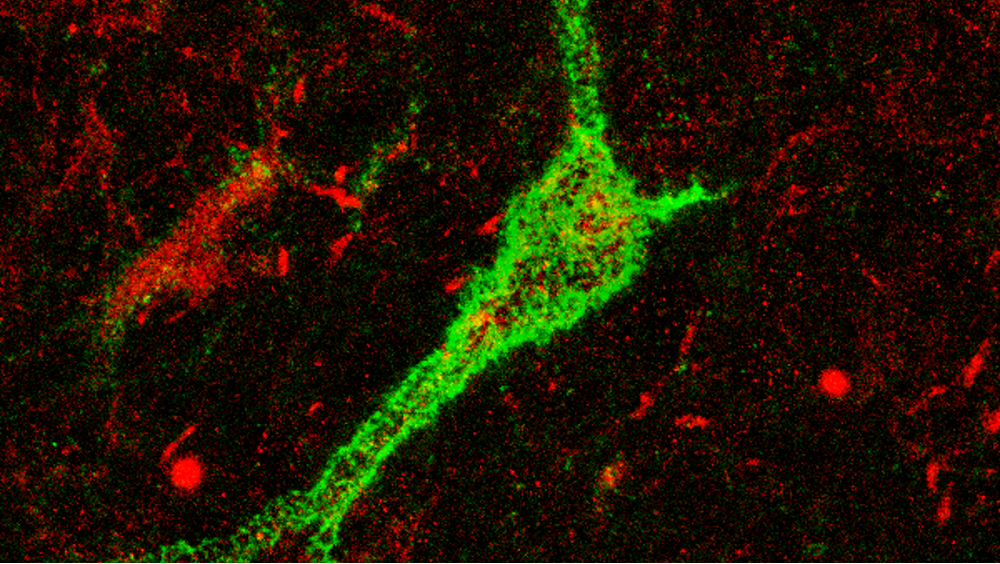
Imagine the brain as a symphony, where each type of neuron plays its part in creating harmony. Inhibitory neurons, such as Parvalbumin-expressing (PV+) cells, help maintain balance in the brain by regulating neuronal activity and preventing overexcitement. They are crucial players in maintaining brain rhythm in the thalamic reticular nucleus (TRN) and habenula—regions linked to the processing of information from multiple sensory modalities, emotions and other important cognitive functions. Our recent research at the University of Valencia, published in Translational Psychiatry, sheds light on how early-life aversive experiences and neurodevelopmental perturbations affect the inhibitory circuits and may contribute to the development of psychiatric disorders as schizophrenia.
What Triggers Changes in Brain Development?
The experiences we encounter early in life impact brain development. They influence how neurons mature and establish connections. Stress or trauma during childhood does not just leave immediate effects; they shape the definitive structure and connectivity of neurons. These experiences, particularly when combined with neurodevelopmental alterations, may increase the risk of developing psychiatric disorders. The effects of adverse experiences in early life are particularly intense on brain regions that have a protracted development, such as the prefrontal cortex, but also the habenula and the thalamus. These regions end the construction of their circuits during infancy or even the adolescence. Consequently, the adverse experiences not only affect them directly during early life, but also influence their final assembly. The inhibitory circuits in these regions of protracted development are the last to mature. For this reason, different types of inhibitory neurons, especially those expressing PV, are significantly vulnerable during early life and are, therefore, strongly affected by early-life aversive experiences.
Why Focus on PV+ Neurons in Schizophrenia Research?
Schizophrenia has long been associated with changes in PV+ neurons. While much of the research has focused on PV+ neurons in the cortex, our study takes a broader look, exploring other important areas often overlooked: the thalamic reticular nucleus (TRN) and the habenula. These regions are key for processing sensory information, regulating emotions, and motivating behavior - all of which can be disrupted in schizophrenia. However, PV+ neurons do not work alone; their function and connectivity is regulated by perineuronal nets (PNN), specialized regions of the extracellular matrix that surround these cells. These structures form a protective and supportive barrier around neurons, helping to regulate their activity and synaptic connections. The physiology of PV+ cells is also influenced by other factors. Plasticity-related molecules like PSA-NCAM and NMDA receptors, which are essential for synaptic plasticity and inter-neuronal communication, significantly shape the function of PV+ neurons. Moreover, glial cells, particularly microglia, are also involved in regulating PV+ neurons.
What We Discovered: The Double-Hit Model of Schizophrenia?
In our study, we used a double-hit model of schizophrenia in mice, combining two key factors: exposure to an NMDA receptor antagonist in early postnatal life, which causes subtle neurodevelopmental alterations, and post-weaning social isolation as a paradigm of early-life aversive experience. Our findings revealed several significant changes. Mice subjected to isolation displayed notable alterations in exploration, behavior regulated by the thalamus and the habenula, indicating increased anxiety, as mice engaged less with their environment and spent less time exploring new spaces. We observed significant changes in PV+ neurons and their PNN. Specifically, there was a decrease in the expression of PV and some components of PNN in the TRN and a decrease in the number of PV+ cells and PNN in the habenula, suggesting alterations in the physiology of these cells and their connectivity. Additionally, the density and area occupied by NMDA receptors in PV+ neurons in the TRN were reduced, indicating impaired synaptic plasticity. Conversely, we found that social isolation significantly influenced the expression of plasticity-related molecules in the TRN, with a marked increase in PSA-NCAM in the dorsal region. We also observed a significant decrease in the area occupied by microglial cells in the habenula following perinatal NMDA receptor antagonist treatment.
Why This Research Matters?
The results show how early-life aversive experience and neurodevelopmental alterations can reshape the brain’s inhibitory networks and impact their integrity and plasticity, particularly in regions like the TRN and habenula. Our findings highlight these diencephalic regions as important targets for events altering the last stages of neurodevelopment occurring during early postnatal life, which may contribute to the development of psychiatric disorders such as schizophrenia. This is a very complex disorder, in which both neurodevelopmental alterations and environmental risk factors, such as adverse experiences in early life, play a crucial role.
Looking Forward
Research on diencephalic regions, such as the thalamus and the habenula, and their inhibitory networks in psychiatric disorders is still evolving. As scientists continue to explore these connections, we expect that these insights will improve our understanding of the biological basis of these diseases, especially schizophrenia, and reveal how early experiences can have lasting effects on our brain health.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Jun 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in