
The carbon dioxide electroreduction reaction (CO2RR) powered by renewable electricity offers a promising route to generate valuable chemicals and fuels.1 However, current CO2RR systems – typically relying on cathodic CO2RR coupled with the anodic oxygen evolution reaction (OER) – suffer from high full-cell voltages, limited CO2 utilization, and, in cases, carbonate formation due to the use of alkaline/neutral media.2-8 Each of these leads to energy penalties when considering the overall system.
CO2RR in acidic media addresses the CO2 loss issue resulting from carbonate formation and crossover in alkaline/neutral media during reaction.9-12 However, hydrogen evolution reaction (HER) under acidic media is kinetically favored relative to CO2RR, leading to low selectivity towards carbon-based products in CO2RR.6,9,10 Thus, to achieve high CO2 utilization efficiency practically, one must suppress HER and promote Faradaic efficiency (FE) to desired products in acidic CO2RR.
As noted above, CO2RR systems today typically rely on OER on the anode, which has a standard potential of 1.23 V vs. the reversible hydrogen electrode.14 Replacing OER with another anodic reaction that requires a lower thermodynamic potential offers to decrease the full-cell voltage and thus the electricity consumption of CO2RR system. It also provides a means to electroproduce economically valuable products at the anode.
Here we report an efficient electrocatalysis system with low energy consumption: cathodic CO2-to-CO in acidic media coupled to anodic allyl alcohol oxidation reaction (AOR) to acrolein (a precursor in the manufacture of acrylic acid), where the cathode chamber and anode chamber are separated by the cation exchange membrane (CEM). In this design, using acidic catholyte and CEM circumvents the CO2 loss arising from the carbonate formation and crossover of carbonate ions from the cathode to anode (Figure 1a below).
In cathodic CO2RR, we lever the K+ strategy reported previously,11 and achieve a CO FE of (96 ± 1)% on an Ag cathode prepared by sputtering a layer of Ag on a carbon-based gas diffusion layer. In anodic AOR, through anode catalyst screening and reaction condition optimization, we achieve an acrolein FE of (85 ± 1)% on a Pt anode at a current density of 100 mA cm−2. We reach a CO2 single pass utilization (SPU) of 84% in this CO2RR-AOR system, fully a 6× improvement compared to the best values reported in prior acidic CO2RR-to-CO studies above 100 mA cm−2.7,8,11,15,16 At 100 mA cm−2, the average full-cell voltage of this paired electrolysis system is 3.2 V during 10 h operation, and the energy consumption for producing 1 kg of CO is 44 MJ; these represent a decrease in voltage of 0.7 V and a reduction of 1.6× in energy consumption relative to the most energy-efficient prior reported acidic ambient-temperature CO2-to-CO studies (Figure 1b).
This thus points to strategies for CO2RR systems with lowered energy consumption.
If you are interested in our work, you may find the full paper here: https://www.nature.com/articles/s41893-024-01363-1
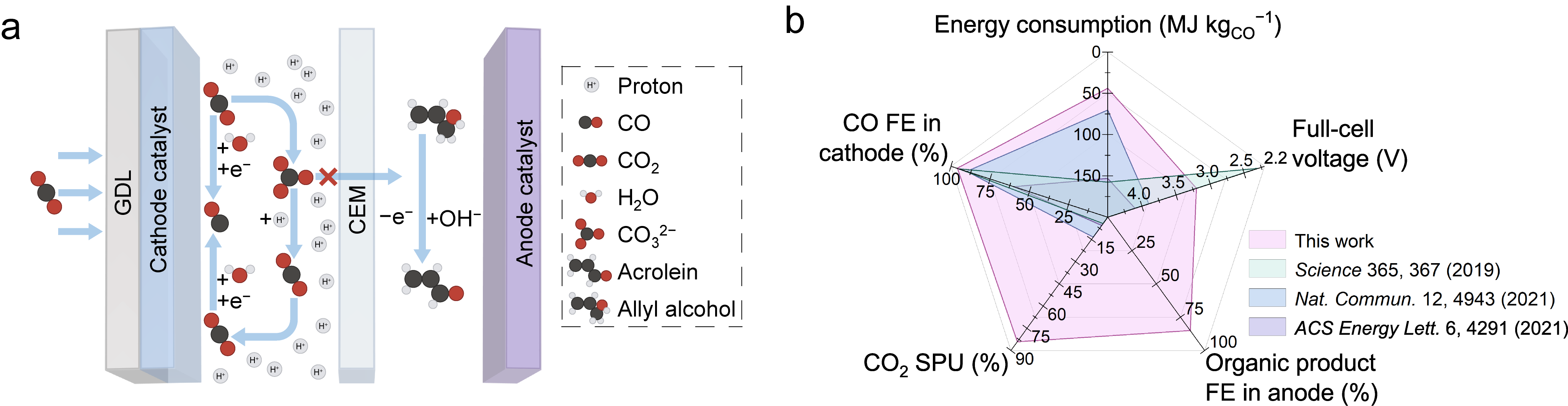
Figure 1. a, Schematic of a paired electrolysis system consisting of CO2-to-CO at cathode and allyl alcohol-to-acrolein at anode. b, Comparison of product selectivities at cathode and anode, full-cell potential, CO2 SPU, and energy consumption in this work with those of state-of-the-art CO2-to-CO ambient-temperature electrolysis systems.
References:
- Jhong, H.-R., Ma, S. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Opin. Chem. Eng. 2, 191−199 (2013).
- Stephens, I. E. L. et al. 2022 Roadmap on low temperature electrochemical CO2 J. Phys. Energy 4, 042003 (2022).
- Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Catal. 1, 748−755 (2018).
- Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Chem. 13, 33−40 (2021).
- Wang, X. et al. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag–Ru–Cu catalyst. Energy 7, 170−176 (2022).
- Ooka, H., Figueiredo, M. C. & Koper, T. M. Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Am. Chem. Soc. 33, 9307–9313 (2017).
- Monteiro, M. C. O., Philips, M. F., Schouten, K. J. P. & Koper, T. M. Efficiency and selectivity of CO2 reduction to CO on gold gas diffusion electrodes in acidic media. Commun. 12, 4943 (2021).
- Yang, K. et al. Cation-driven increases of CO2 utilization in a bipolar membrane electrode assembly for CO2 ACS Energy Lett. 6, 4291−4298 (2021).
- Wu, Y. et al. Electrochemical CO2 reduction using gas diffusion electrode loading Ni-doped covalent triazine frameworks in acidic electrolytes. 88, 359–364 (2020).
- Bondue, C. J., Graf, M., Goyal, A. M. & Koper, T. M. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 J. Am. Chem. Soc. 143, 279–285 (2021).
- Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
- Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Catal. 5, 564−570 (2022).
- Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Chem. 13, 33−40 (2021).
- Verma, S., Lu, S. & Kenis, P. J. A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Energy 4, 466−474 (2019).
- Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Catal. 5, 268−276 (2022).
- Jiang, Z., Zhang, Z., Li, H., Tang, Y., Zao, J., Zheng, H. & Liang, Y. Molecular catalyst with near 100% selectivity for CO2 reduction in acidic electrolytes. Energy Mater. 13, 2203603 (2023).
Follow the Topic
-
Nature Sustainability

This journal publishes significant original research from a broad range of natural, social and engineering fields about sustainability, its policy dimensions and possible solutions.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcement

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in