Efficient Self-Assembly of Heterometallic Triangular Necklace with Strong Antibacterial Activity
Published in Chemistry

The paper in Nature Communications is here: https://doi.org/10.1038/s41467-020-16940-z
Mechanically interlocked molecules (MIMs) such as catenanes, rotaxanes and molecular knots have attracted great interest in the field of supramolecular chemistry due to their innately topologically nontrivial architectures, thus always challenging the imagination and skills of synthetic chemists. Molecular necklaces, as important members of the MIM family, are derived from catenanes, in which three or more side rings as molecular “beads” are threaded onto a central ring as molecular “chains”. It's worth noting that, though a major breakthrough in MIMs regarding to their impressive structures as well as well-developed synthetic strategy has been achieved, the focus of the chemistry of MIMs, especially for molecular necklaces, has still stayed on molecular design and synthesis stage. Studies involving their applications are hardly seen at present in this area.
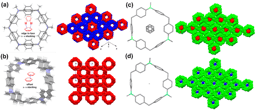
Our group in East China Normal University is specialized in supramolecular self-assembly at different scale based on platinum chemistry, mainly including platinum(II)‐pyridine coordination-driven self-assembly and platinum acetylide rotaxane dendrimers. Coordination-driven self-assembly has proven to be a powerful approach to construct a diverse range of supramolecular architectures including metallacycles, metallacages, and metal-organic frameworks (MOFs), through the combination of a range of diverse functional ligands and complementary metal salts. The intrinsic nature of coordination-driven self-assemblies, including their high dynamic metal–ligand coordination bonds, diverse metal ions, positively charged molecular nature, together with their highly tunable coordination geometries, endows them with great merits in biomedical applications. Under this premise, in this paper, an unprecedented heterometallic triangular necklace 1 containing Cu and Pt metals has been designed and synthesized via highly efficient coordination-driven self-assembly between pseudorotaxane D1 consisting of two functional ligands of pyridine and phenanthroline and diplatinum(II) acceptor unit A1 in nearly quantitative yield (Figure 1). The elegant structure of necklace 1 is successfully determined by X-ray crystallographic analysis, revealing that the finely arranged triangular necklace 1 has two racemic enantiomers in its solid state with intriguing packing motif.
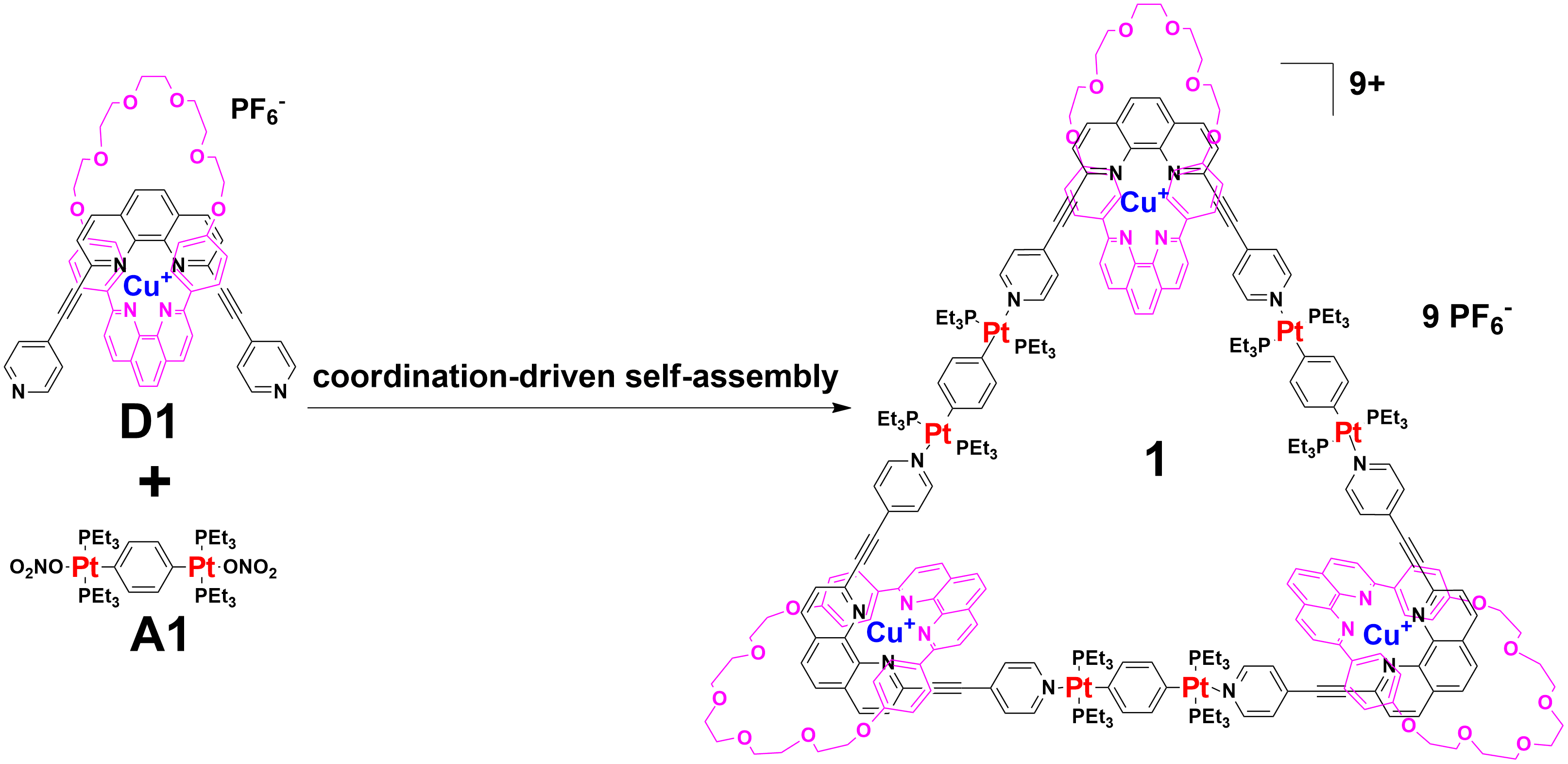
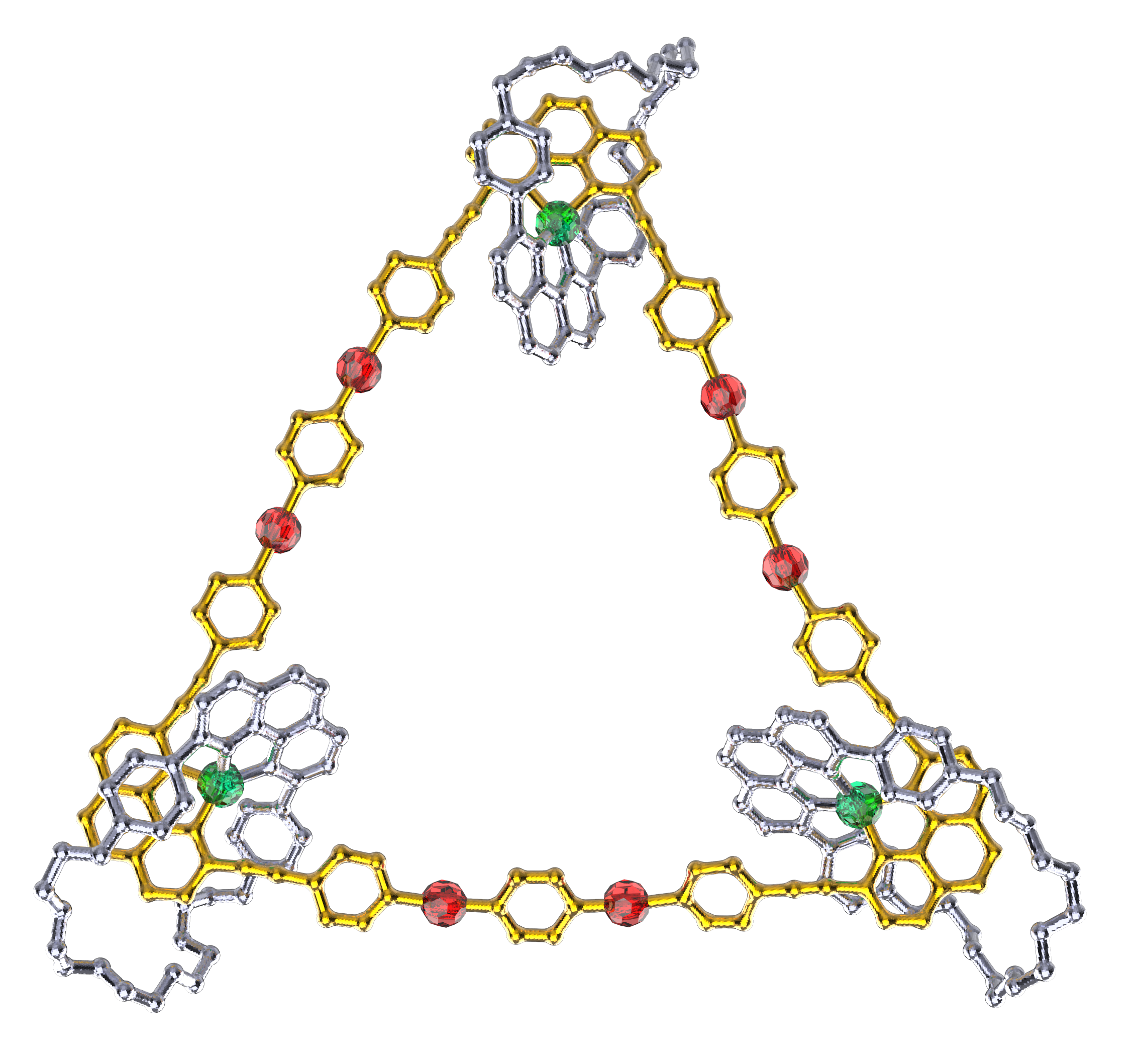
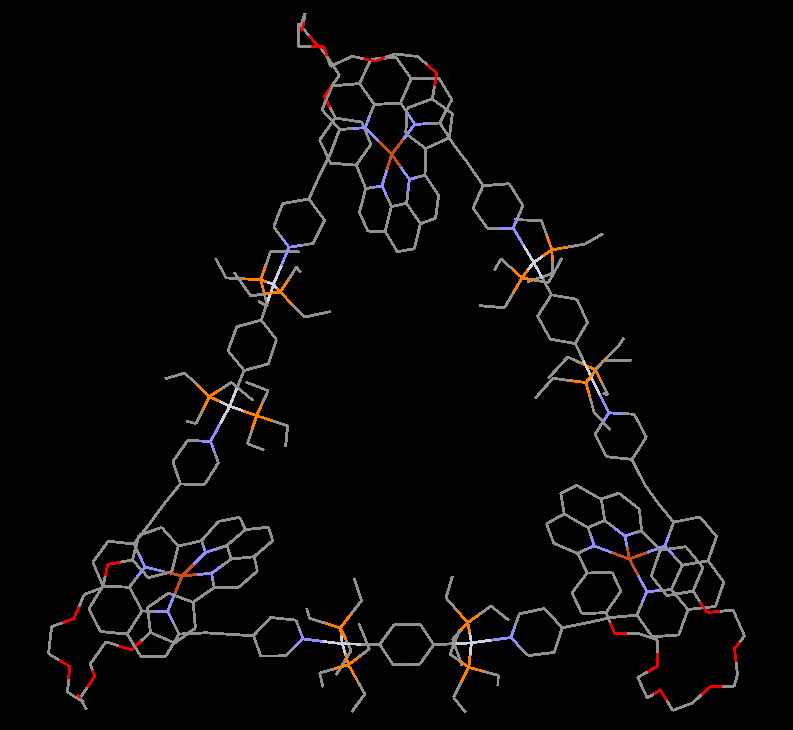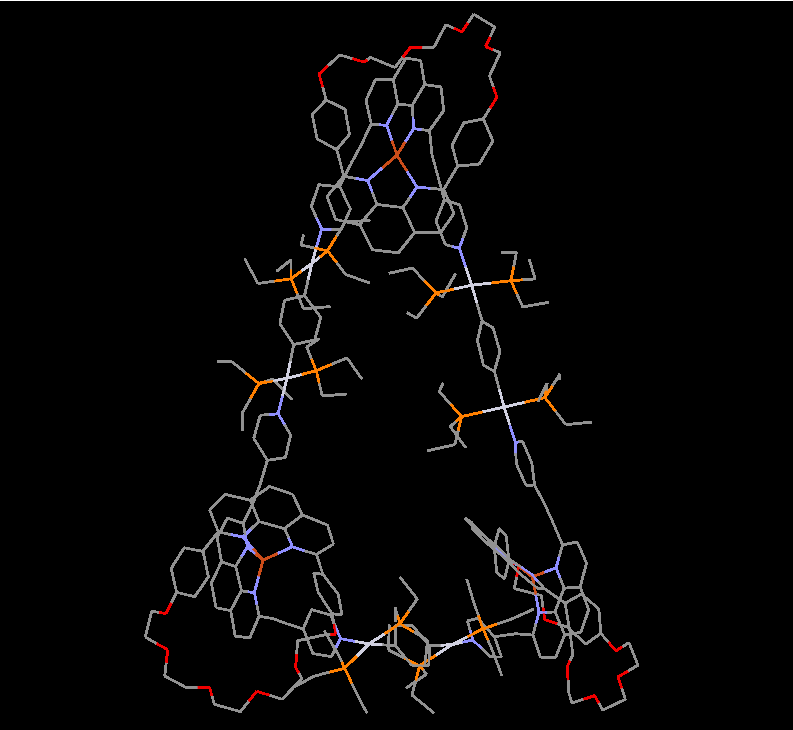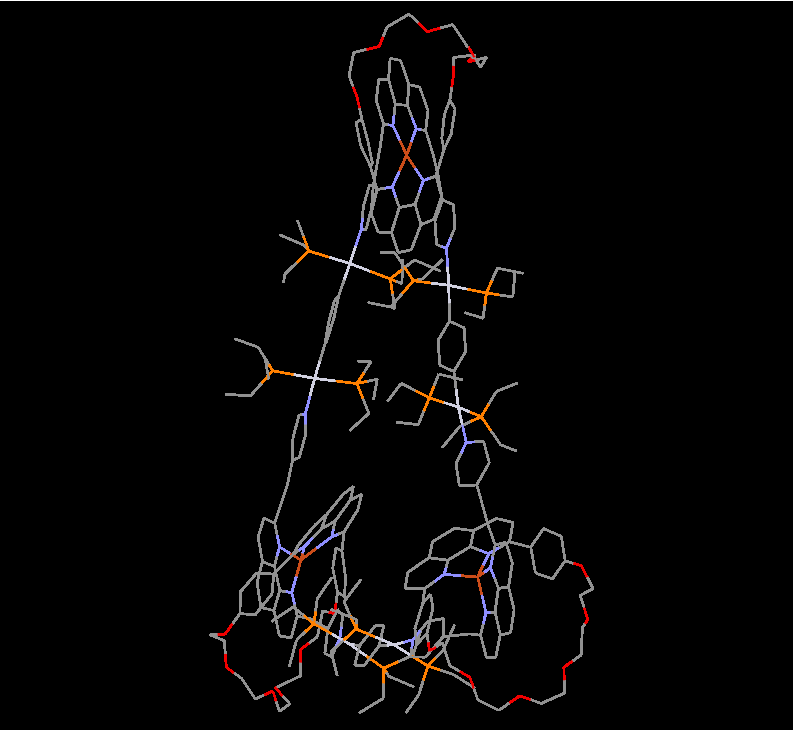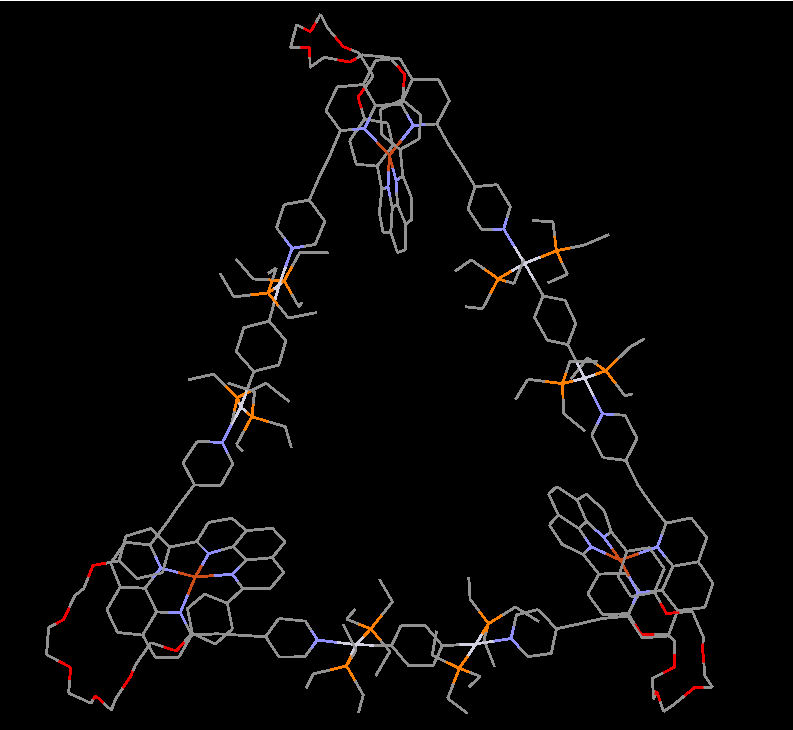
Figure 1 Coordination-driven self-assembly of necklace 1
In collaboration with Prof. Qilin Yu from Nankai University, we have successfully demonstrated that the existence of two different metal centers not only facilitates the successful construction of necklace 1 but also endows it with superior nuclease properties and activities. More strikingly, the presence of the Cu(I) center and platinum(II), together with the coordination-based triangular framework, facilitates binding of necklace 1 to the cell surface components and disruption of the cell wall/plasma membrane in the bacterial cells (Figure 2). Consequently, the necklace 1 exhibited excellent bactericidal activity against both standard and clinical drug-resistant pathogens that are severely threatening human health, e.g., multidrug-resistant Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. Further investigations revealed that the superior antibacterial activity of necklace 1 is mainly attributed to the highly positively charged nature, the possible synergistic effect of heterometals in the necklace, and the improved stability in culture media. The “threading-followed-by-ring-closing” approach combining with coordination-driven self-assembly would allow us to further construct more complicated molecular necklaces in the future. Moreover, the promising DNA cleavage and antibacterial activities obtained herein would also attract broad interests of chemists, material scientists, and biomedical scientists, and provide directions for future chemical design in the field of supramolecular chemistry.
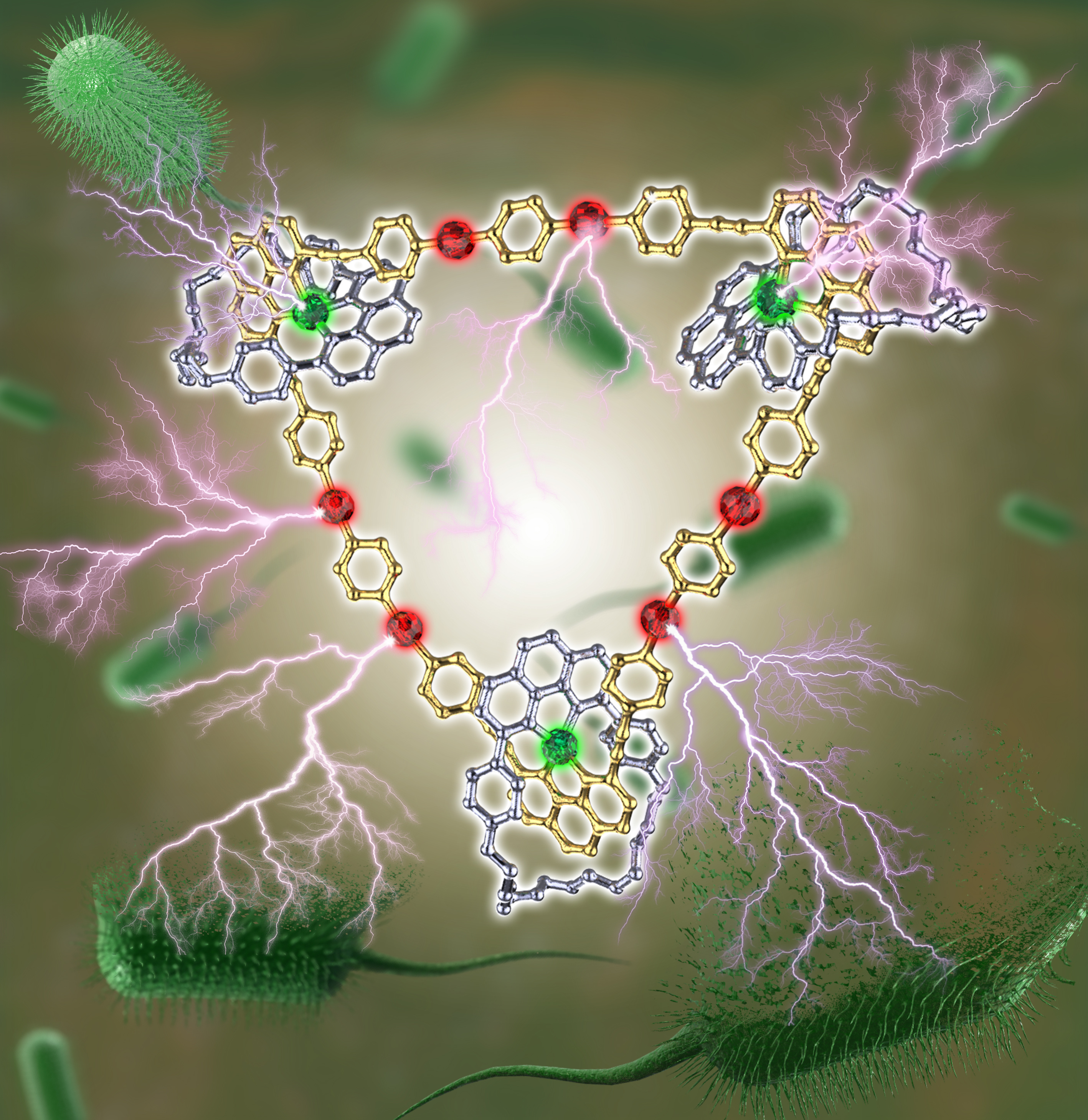
Figure 2 Cartoon representation of antibacterial action of necklace 1
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in