Efficient solvent- and hydrogen-free upcycling of high-density polyethylene into separable cyclic hydrocarbons
Published in Chemistry
Why choose solvent- and hydrogen- free upcycling method?
Plastic pollution is a planetary threat, while most of the current recycling processes are challenged by limited economic incentives1. A promising alternative is chemical upcycling, while typical approaches consume large amounts of co-reactants such as H2 and olefins which are difficult to match the scale of plastic waste2,3. Therefore, we tried to develop a socially sustainable and economically viable method for plastic upcycling without using consumable materials such as co-reactants or solvents.
Why HZSM-5?
We looked through previous research for inspiration on solvent- and hydrogen- free method that breaks down high-density polyethylene (HDPE), one of the most widely used polymer. A report about Pt on γ-Al2O3 (Pt/γ-Al2O3) attracted us for its good catalytic performance towards upcycling polyethylene into valuable long-chain alkylbenzenes4. In this process, polyethylene hydrogenolysis into lower molecular weight by H2 molecules which were in-situ generated from ring closure and dehydroaromatization, thereby not requiring external H2. This result is very interesting and enlightening, so we repeated the experiment and tried some modifications. However, we found that Pt loaded with different crystal forms (α- and γ-) of Al2O3 showed a big difference in catalytic performance towards polyethylene upcycling. This phenomenon aroused our curiosity.
Were there some intrinsic mechanisms not discovered? One day, while analyzing the ICP-AES data of another student in our group, we noticed that different crystal forms (α- and γ-) of Al2O3 contained different percentage compositions of Si due to different synthetic methods. Si modification on Al2O3 might create additional Brønsted acid sites. It hit us that maybe trace of Brønsted acid sites originated from Si-OH-Al in Al2O3 matters.
So, we turned our attention to metal supported zeolite-based catalysts which have abundant Si-OH-Al sites. The first batch of tests was conducted with Ru/USY. As expected, the average mass activity of Ru/USY was around 9 times as high as the best value of Pt/γ-Al2O3 reported in solvent/hydrogen-free plastic upcycling. However, with the ultraviolet-fluorescence spectrum, we detected some polycyclic aromatic hydrocarbons (PAHs) in the solid residue after plastic upcycling, which may lead to carbon deposition on the active sites of the catalyst. Meanwhile, the gel permeation chromatography (GPC) profile showed that the remaining polymer has a much lower molecular weight with lower entropy. Considering that this reaction is entropy driven, the reactivity of solid residue decreased. This phenomenon raised our concerns about the robustness of Ru/USY.
According to the pore confinement effect, zeolites with smaller pore diameters should be applied in order to prevent the desorption of polymer chain. At the same time, the space of cages in zeolites should be smaller to alleviate the carbon deposition of the catalyst. Therefore, a more effort on newly-focused Ru/HZSM-5(300) was made, and we found surprisingly that it behaved far more productively than Ru/USY.
This time, we briefly went closer to desired catalytic performance of HDPE upcycling, and what to do is more confirm and more precise explanation on the mechanics.
What’s the performance of Ru/HZSM-5(300)?
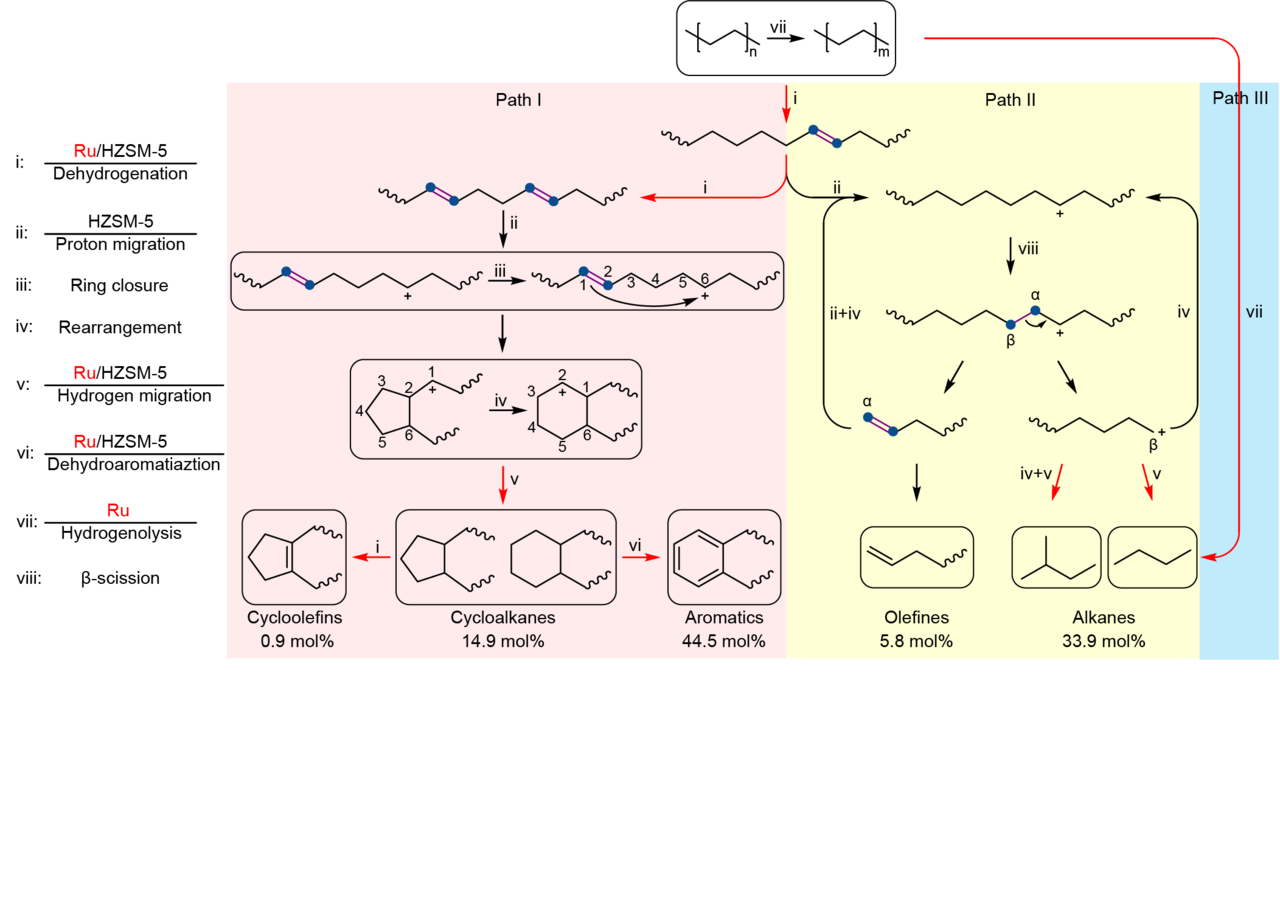
The catalyst incorporated Ru nanoparticles supported on HZSM-5 with a Si/Al ratio of 300, denoted as Ru/HZSM-5(300). Notably, the average mass activity of Ru/HZSM-5(300) reached 290.0 mgHDPE gcat-1 h-1 at 280 oC for 24 h, being 18 times as high as the best value (16.0 mgHDPE gcat-1 h-1) ever reported in solvent/hydrogen-free plastic upcycling. HDPE powders were selectively converted into separable products where the distribution of linear (C1-C6) and cyclic hydrocarbons (C7-C15) deviated so largely as to be feasibly separated. The major components are valuable monocyclic hydrocarbons with a high selectivity of up to 60.3 mol%, containing cycloalkanes (14.9 mol%), cycloolefins (0.9 mol%), and aromatics (44.5 mol%).
What’s the mechanism of the reaction?
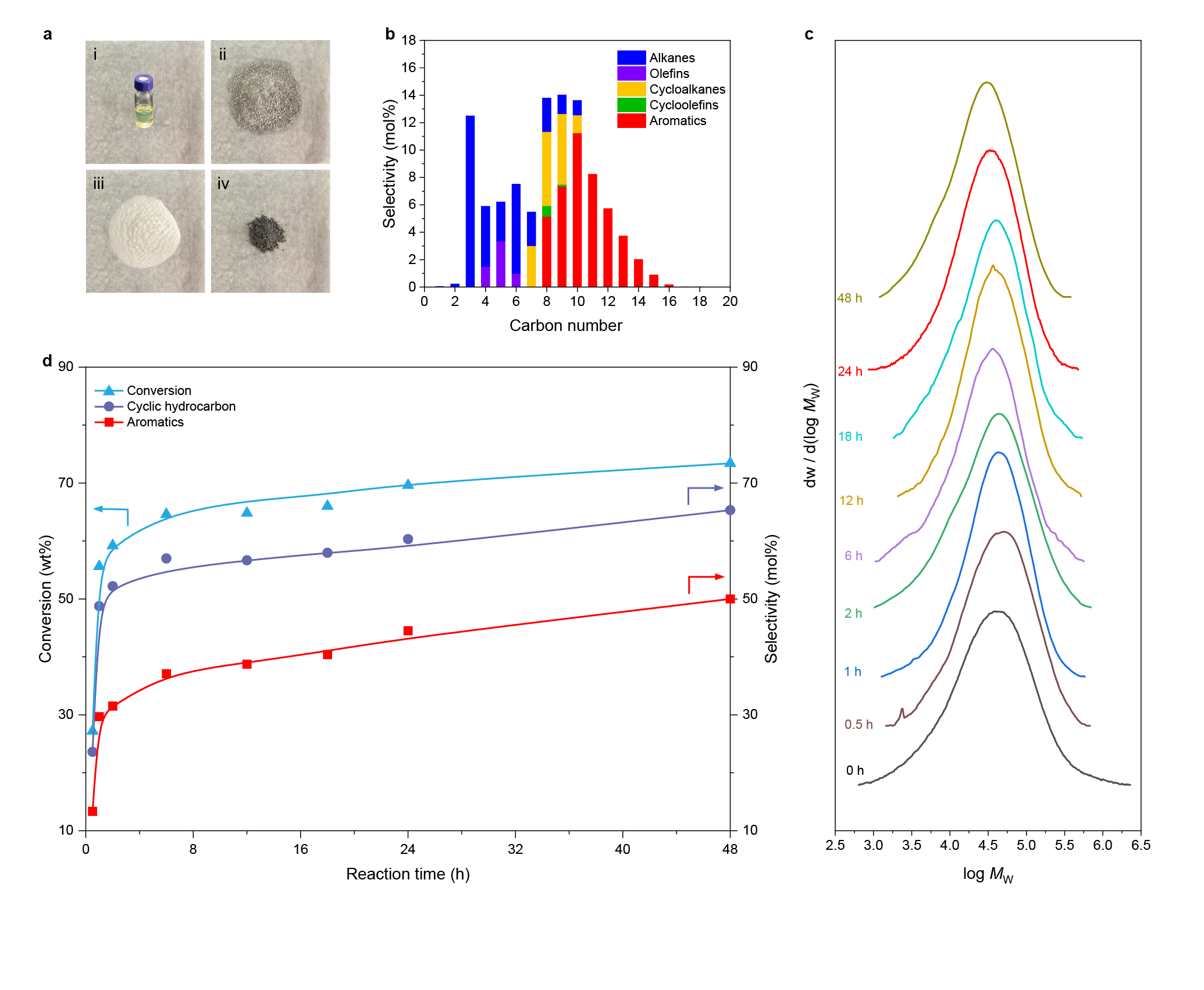
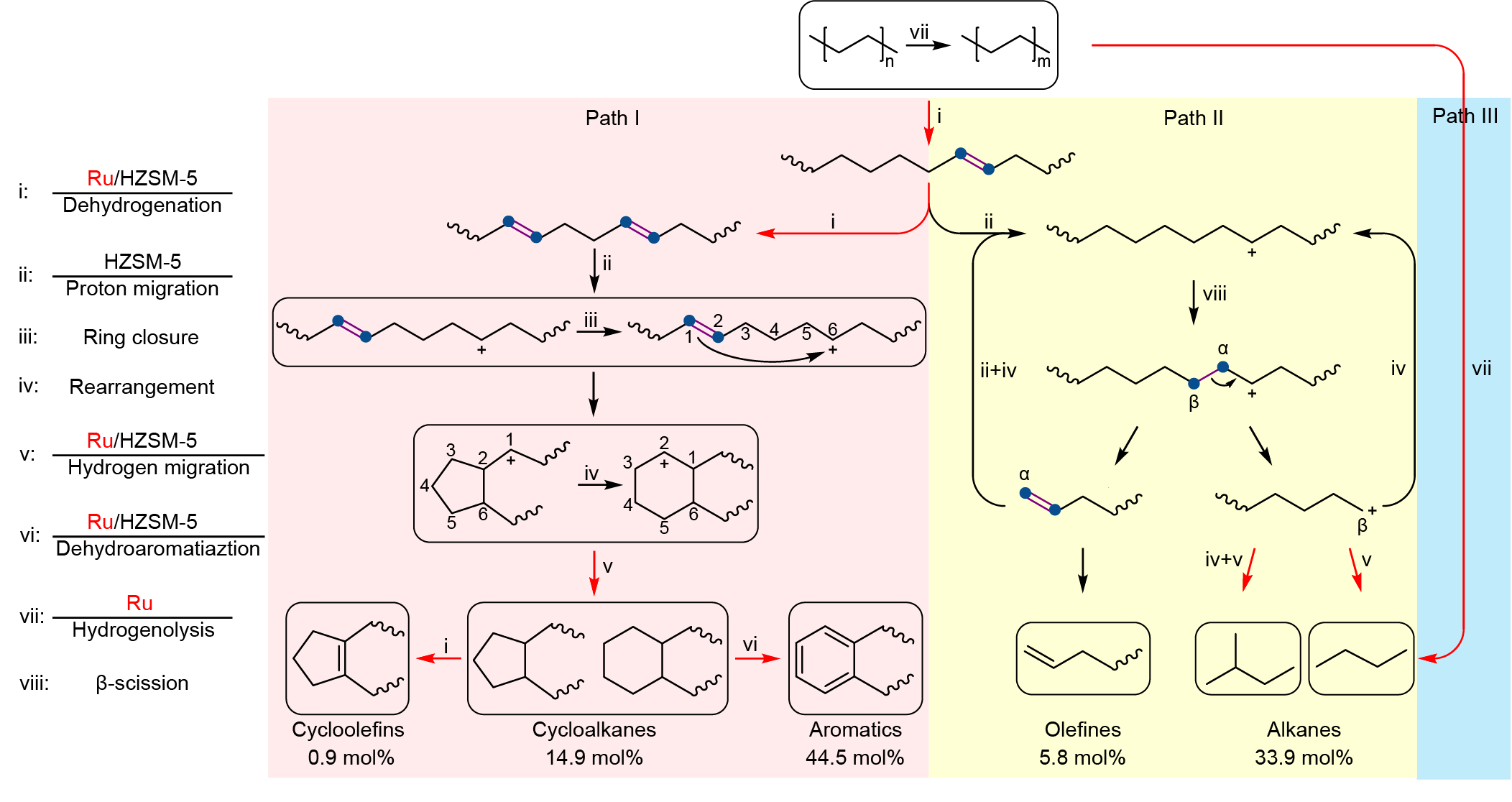
Further mechanistic studies revealed that the high activity was attributed to the synergy between Ru and HZSM-5. Specifically, a polymer chain is dehydrogenated over Ru and acid sites in HZSM-5 into C=C bonds, some of which transform into carbenium ions on acid sites via proton transfer. The optimization of Ru sites and acid sites facilitates the cyclization process which requires the simultaneous existence of a C=C bond and a carbocation on a molecular chain with a proper distance. Moreover, the small pore volume of HZSM-5 impedes the formation and diffusion of fused aromatic rings that are responsible for coking, thereby rendering high stability towards HDPE pyrolysis.
How channel works in HDPE upcycling?
We investigated the pore structure of zeolites in HDPE upcycling. For HZSM-5, the space of cages in HZSM-5 only accommodate the formation of monocyclic aromatics, thus prevent the carbon deposition. At the same time, due to the different residence times of linear hydrocarbons with different carbon number in the channel, Ru/HZSM-5 enabled selective upcycling of HDPE into a separable distribution of linear and cyclic hydrocarbons.
In conclusion, we demonstrated that Ru/HZSM-5(300) enabled selective upcycling of HDPE into a separable distribution of linear and cyclic hydrocarbons in the absence of solvent or external H2. The valuable monocyclic hydrocarbons accounted for 60.3 mol% in gaseous and liquid fractions. Moreover, the catalyst showed high stability and high robustness for the upcycling of PE in different commercial grades. This work offers a potential route to recycle plastic waste without any consumable materials. The value-added products are likely to provide more economic incentives for plastic recycling. Moreover, our findings advance the understanding of catalytic mechanisms during plastic upcycling.
More details of this work can be found here: “Efficient solvent- and hydrogen-free upcycling of high-density polyethylene into separable cyclic hydrocarbons." in Nature Nanotechnology, https://doi.org/10.1038/s41565-023-01429-9.
Reference
- Rahimi, A. & Garcia, J. M. Chemical recycling of waste plastics for new materials production. Rev. Chem. 1, 0046 (2017).
- Conk, R. J. et a Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 377, 1561-1566 (2022).
- Wang, N. M. et a Chemical Recycling of Polyethylene by Tandem Catalytic Conversion to Propylene. J. Am. Chem. Soc. 144, 18526-18531 (2022).
- Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437-441 (2020).
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
niu