Electrochemical Deoxygenative Amination of Stabilized Alkyl Radicals from Activated Alcohols
Published in Chemistry
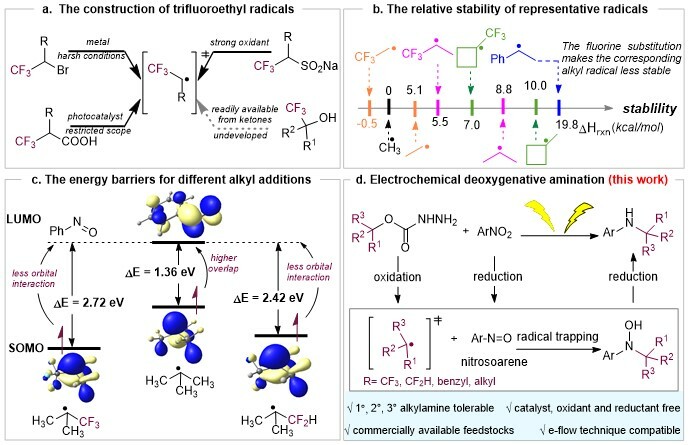
Amines are among the most important organic compounds in materials science and pharmaceuticals, and thus the development of efficient methods for their construction has been a subject of considerable interest to organic chemists. A variety of innovative approaches for alkylamine functionalization have been developed, the most general methods to prepare alkylamines are reductive amination, Buchwald-Hartwig amination, Ullman-type reaction and Chan-Lam amination. Nevertheless, the efficient construction of C(sp3)-N bonds remains highly challenging.
The research group led by Professor Yi Wang at Nanjing University has long been committed to the study of C(sp3)-N bond construction and has made a series of research advancements in this area (Sci. China Chem. 65, 678-685 (2022), ACS Catal. 12, 11071–11077 (2022), Green Chem. 25, 7084–709 (2023)). Recently, Professor Yi Wang have developed a general deoxygenative C−N coupling reaction of activated alcohols and nitroarenes. Alkyl amines with α-CF3, α-CF2H and benzyl substituents can be readily accessed under the electrochemical conditions. The mismatched reactivity of alkyl radicals and nitrogen sources has been addressed by paired electrolysis. The practicality of this method with electrochemical continuous flow technique has been demonstrated.
Article link:https://doi.org/10.1038/s41467-024-50596-3
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in