Electrochemically mediated carbon capture in salt-concentrated aqueous media
Published in Chemistry

Anthropogenic carbon dioxide emissions present a severe challenge to our society. One of the foremost mitigation strategies involves carbon capture, particularly from large stationary emission sources, followed by sequestration or utilization. The incumbent technology for carbon capture is thermal amine scrubbing, which is challenged by the substantial energy demand associated with the regeneration step, sorbent degradation, corrosion, environmental concerns, and large footprint.
Our group aims to enable new carbon capture technologies using renewable electricity. For example, over the years, we have successfully developed the concept of electrochemically mediated amine regeneration (EMAR) in lieu of the traditional thermal swing method (Energy Environ. Sci., 2013, 6, 2505-2517). Another way to achieve electrochemically mediated carbon dioxide separation is by using redox-active sorbents, such as quinones, that undergo changes in their carbon dioxide binding affinity as they progress through an electrochemical cycle (Figure 1). We have recently demonstrated a working prototype of such concept in a parallel passage contactor configuration using polyanthraquinone-carbon nanotube composites in ionic liquid (Energy Environ. Sci., 2019,12, 3530-3547). By being electrically driven, these systems can be controlled precisely to reduce energy losses, are modular and thus easy to implement in a variety of locations, and possess great adaptability to the multi-scale nature of carbon capture.
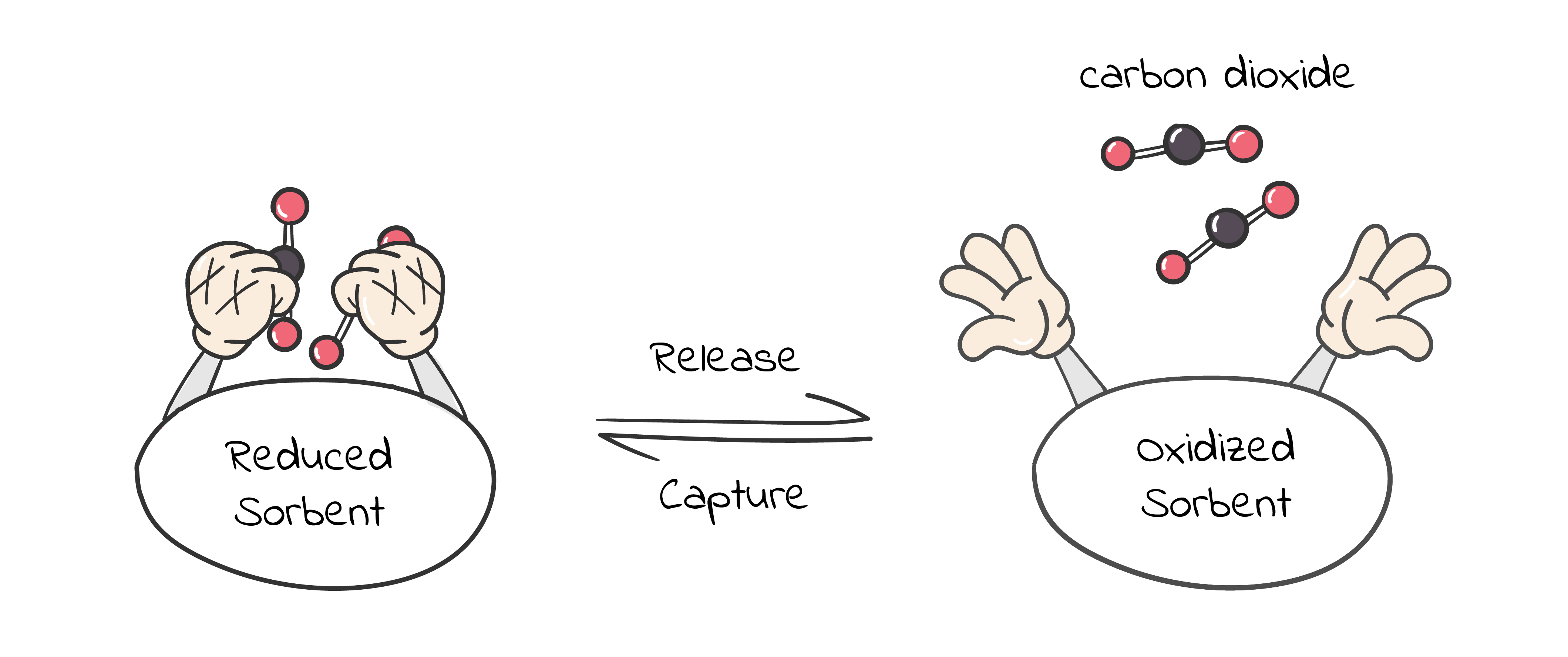
Many electrochemically mediated carbon dioxide separation processes using redox-active sorbents need to be operated using mainly flammable, toxic, aprotic organic electrolytes, posing a barrier toward practical implementation. An aqueous electrolyte is the ideal medium for large-scale electrochemical systems, offering distinct advantages in cost, safety, environmental benignity, and ease of device construction. However, aqueous electrolytes are incompatible with redox-active carbon dioxide sorbents due to serious challenges such as the narrow electrochemical stability window of water, complex carbon dioxide speciation upon dissolution, rapid evaporative loss, and importantly, the presence of various competing reactions.
In our new study, by drawing inspiration from the recent breakthrough in salt-concentrated electrolytes for battery applications, we developed an aqueous electrolyte formulation that is fully compatible with redox-active carbon dioxide sorbents. Importantly, quinone-based sorbents are thermodynamically more reactive towards carbon dioxide and less prone to competing reactions in salt-concentrated aqueous electrolytes compared to in its dilute counterpart, due to the modified dielectric environment, as verified by both experiments and theoretical calculations. Correspondingly, we achieved continuous carbon capture-release operations with outstanding capacity, stability, efficiency, and electrokinetics under realistic gas compositions, advancing electrochemical carbon separation further towards practical applications.
All the details of this study can be read in our Nature Communications article, which can be downloaded from the link below:
https://www.nature.com/articles/s41467-020-16150-7
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in