Emerging racial disparities among Medicare beneficiaries and Veterans with metastatic castration-sensitive prostate cancer
Published in Cancer

Previous studies have shown racial disparity in the treatment of Black patients versus their White counterparts with localized prostate cancer or metastatic castration-resistant prostate cancer (mCRPC)1-7. Retrospective and prospective analyses suggest that with equal access to treatment, Black and White patients with mCRPC may benefit similarly8-12, despite population based evidence that Black men are more likely to develop and die from prostate cancer at a younger age than White men13. However, analyses in patients with earlier stages of disease including metastatic castration-sensitive prostate cancer (mCSPC) are dated and based on prior guideline recommendations14-16. Treatment intensification (TI) consisting of the addition of docetaxel and/or novel hormone therapy to androgen deprivation therapy has evolved over the last 9 years, demonstrating consistently greater improved survival for patients with mCSPC, and has been widely adopted by consensus guidelines17-20. This study aimed to determine if racial disparity exists in treatment and clinical outcomes specifically among patients with mCSPC since the acceptance of TI as standard of care.
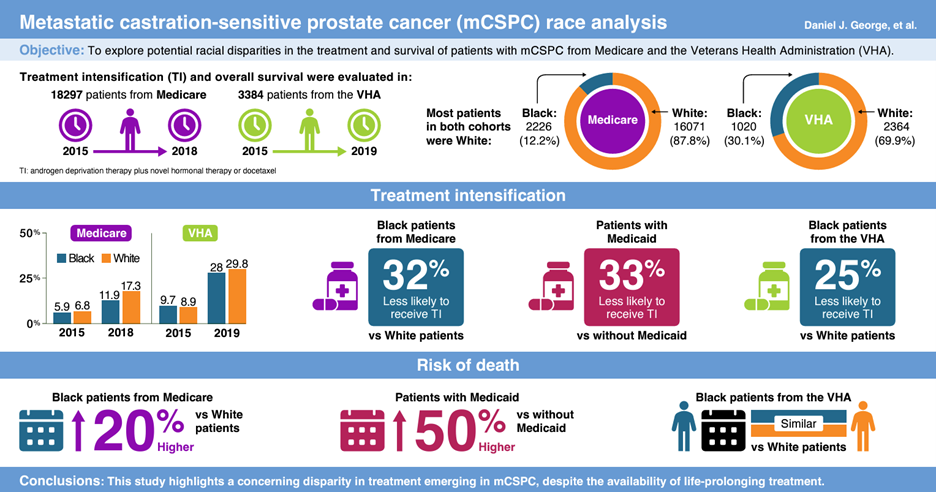
In our real-world evidence study21, we collected data from two large US national databases: Medicare, which includes low-income Medicaid patients, and the Veterans Health Administration (VHA; Figure 1). Data were obtained from patients with mCSPC in Medicare records from January 2015 to December 2018 and in the VHA records from January 2015 to June 2019. Data from 18,297 patients (n=2226 Black patients; n=16,071 White patients) in Medicare were analyzed, of whom 2339 (n=898 Black patients; n=1441 White patients) were enrolled in Medicaid, where eligibility is largely based on income status. Data were analyzed from an additional 3384 patients (n=1020 Black patients; n=2364 White patients) enrolled in the VHA.
Specifically, we looked at whether the use of TI and patient survival were impacted by race (Black vs White) and income (Medicaid vs non-Medicaid insurance). Despite consensus guideline recommendations, even in the most recent years evaluated, over two-thirds of all patients enrolled in Medicare and the VHA did not receive TI. However, these discrepancies were even greater when analyzed by race; Black patients were approximately 32% and 25% less likely to receive TI for their mCSPC compared with White patients in Medicare and the VHA, respectively. Interestingly, when analyzed in patients enrolled in Medicaid, there was no difference between the use of TI for Black and White patients. Overall, patients enrolled in Medicaid were less likely to receive TI compared with patients not enrolled in Medicaid. For the more inclusive Medicare population, Black patients had a 20% higher risk of death compared with White patients. For patients enrolled in Medicaid, race was not associated with worse patient survival; however, patients enrolled in Medicaid overall had a 50% higher risk of death compared with patients not enrolled in Medicaid, suggesting that income level negatively influences disease outcome in both races.
In summary, we observed an underutilization of guideline recommended TI among all patients with mCSPC despite consistent evidence for survival benefit. TI was lower for Black patients compared with White patients in both Medicare and VHA, and race was associated with worse survival outcomes among Medicare patients but not VHA patients. Importantly, poverty was also associated with a lower quality of care, as evidenced by less TI and worse overall survival among Medicaid patients compared with non-Medicaid patients. Our study warns of emerging disparities in both treatment and patient outcomes based on race and income. To reverse this trend, it is important for the healthcare community and the public to be aware of, and advocate for, TI in all patients with mCSPC, regardless of race or economic status.
Figure 1. Graphical summary of the research study21.
References
1. Gerhard, R. S. et al. Treatment of men with high-risk prostate cancer based on race, insurance coverage, and access to advanced technology. Urol Oncol. 35, 250–256 (2017).
2. Khan, S. et al. Predictors of Follow-Up Visits Post Radical Prostatectomy. Am J Mens Health. 12, 760–765 (2018).
3. Caram, M. E. V. et al. Factors influencing treatment of veterans with advanced prostate cancer. Cancer. 127, 2311–2318 (2021).
4. Moses, K. A., Orom, H., Brasel, A., Gaddy, J. & Underwood, W., 3rd. Racial/ethnic differences in the relative risk of receipt of specific treatment among men with prostate cancer. Urol Oncol. 34, 415.e7–415.e12 (2016).
5. Mouzannar, A. et al. PD34-03 Racial disparity in the utilization of new therapies for advanced prostate cancer. J Urol. 206, e583 (2021).
6. Mahal, B. A. et al. Getting back to equal: The influence of insurance status on racial disparities in the treatment of African American men with high-risk prostate cancer. Urol Oncol. 32, 1285–1291 (2014).
7. Rude, T. et al. Interaction between race and prostate cancer treatment benefit in the Veterans Health Administration. Cancer. 127, 3985–3990 (2021).
8. Halabi, S. et al. Overall Survival of Black and White Men With Metastatic Castration-Resistant Prostate Cancer Treated With Docetaxel. J Clin Oncol. 37, 403-410 (2019).
9. Sartor, O. et al. Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 23, 517–526 (2020).
10. Riviere, P. et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer. 126, 1683–1690 (2020).
11. George, D. J. et al. A prospective trial of abiraterone acetate plus prednisone in Black and White men with metastatic castrate-resistant prostate cancer. Cancer. 127, 2954–2965 (2021).
12. George, D. J. et al. Survival by race in men with chemotherapy-naive enzalutamide- or abiraterone-treated metastatic castration-resistant prostate cancer. Prostate Cancer and Prostatic Diseases. 25, 524–530 (2022).
13. Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J Clin. 72, 7-33 (2022).
14. Bernard, B. et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer. 123, 1536–1544 (2017).
15. Dess, R. T. et al. Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality. JAMA Oncol. 5, 975–983 (2019).
16. Stern, N. et al. Association of Race and Ethnicity With Prostate Cancer–Specific Mortality in Canada. JAMA Netw Open. 4, e2136364 (2021).
17. Virgo, K. S. et al. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update. J Clin Oncol. 39, 1274-1305 (2021).
18. Lowrance, W. T. et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J Urol. 205, 14–21 (2021).
19. Mottet, N. et al. EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on Prostate Cancer. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2023_2023-03-27-131655_pdvy.pdf (2023).
20. Lowrance, W. et al. Updates to Advanced Prostate Cancer: AUA/SUO Guideline (2023). J Urol. 209, 1082–1090 (2023).
21. George, D. J. et al. Emerging racial disparities among Medicare beneficiaries and Veterans with metastatic castration-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 10.1038/s41391-024-00815-1 (2024). https://www.nature.com/articles/s41391-024-00815-1
Acknowledgments
Medical writing and editorial support were provided by Peter Gray, PhD, and Rosie Henderson, MSc, both of Onyx (a division of Prime, UK), funded by Pfizer Inc and Astellas Pharma Inc.
Funding
This study was sponsored by Pfizer Inc. and Astellas Pharma Inc., the co-developers of enzalutamide.
Daniel J George reports funding from American Association for Cancer Research; grants and personal fees from Astellas Pharma Inc., BMS, Janssen Pharmaceuticals, Novartis, and Pfizer Inc.; personal fees from AstraZeneca Axess Oncology, Flatiron, Merck, Sharp & Dohme, Michael J Hennessey Assoc, Millennium Medical Publishing, Sumitomo Pharma, NCI Genitourinary, Physician Education Resource, Propella Therapeutics, RevHealth, Seattle Genetics, UroGPO, WebMD, and Xcures; grants, personal fees, and non-financial support from Bayer H/C Pharmaceuticals, Exelixis Inc., and Sanofi; grants from Calithera, Capio Biosciences and EMD Serono; personal fees and non-financial support from UroToday.
Follow the Topic
-
Prostate Cancer and Prostatic Diseases

This journal covers all aspects of prostatic diseases, in particular prostate cancer, the subject of intensive basic and clinical research world-wide.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in