Enantioconvergent Sonogashira Coupling: A Radical Way to Chiral C–C Bonds under Copper Catalysis
Published in Chemistry

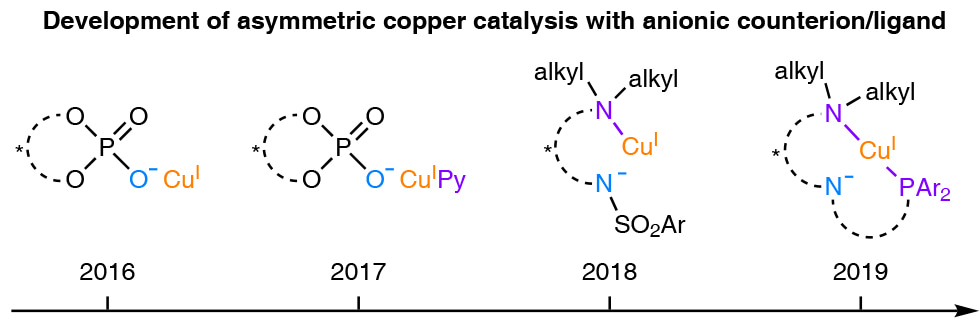
Our group has recently been focusing on developing asymmetric copper catalysis with chiral anionic counterion/ligand for radical-involved transformations. In this respect, we have developed Cu/chiral phosphate catalysts for a series of asymmetric 1,2-difuctionalization of unactivated alkenes. In one particular case, we have found an ancillary pyridine ligand to be essential for highly enantioselective 1,2-alkoxytrifluoromethylation of unactivated alkenes. This result encouraged us to combine a neutral ligand with a chiral anionic counterion/ligand for asymmetric copper catalysis. Subsequently, we identified a cinchona alkaloid-derived sulfonamide as a robust anionic/neutral hybrid ligand for copper to realize asymmetric 1,2-iminoxytrifluoromethylation of unactivated alkenes.
During these studies, we became aware of one salient advantage of such a hybrid ligand over a common anionic counterion/ligand: the neutral coordinating motif could help to efficiently tune the electronic property of the copper center. Thus, very mild oxidative radical precursors, such as usual alkyl halides, would be reducible by a CuI catalyst with an electron-rich hybrid ligand. In this sense, the scope for our asymmetric copper catalysis with chiral anionic counterion/ligand would be greatly expanded.
In order to demonstrate this potential, we have become interested in accomplishing an enantioconvergent Sonogashira C(sp3)–C(sp) coupling of alkyl halides with terminal alkynes. On one hand, the readily occurring Glaser homocoupling of terminal alkynes determines that only mild oxidant would be suitable for achieving high chemoselectivity. On the other hand, the accommodation of most types of alkyl halides would necessitate a sufficient reducing power of the copper catalyst. Accordingly, we have deliberately examined potential hybrid ligands with strongly electron-donating motifs, such as tertiary amine and phosphine. Fortunately, we have found a N,N,P-ligand, a derivative of the Dixon’s ligand, to be superior for the envisioned enantioconvergent Sonogashira C(sp3)–C(sp) coupling.
Thus, racemic (hetero)benzylic, allylic, propargyl, a-aminocarbonyl, and a-cyano alkyl halides readily couple with a variety of terminal alkynes. Of particular note is the accommodation of industrially relevant propyne and acetylene, thus indicating the potential large-scale application of this methodology. More importantly, further one- or two-step transformations after coupling deliver a range of chiral C(sp3)–C(sp2) and C(sp3)–C(sp3) bonds in drug and natural product molecules. This coupling involves readily available alkyl halides and terminal alkynes as substrates, thus avoiding compatibility and/or cost issues associated with organometallic and/or prefunctionalized reagents. In this sense, our enantioconvergent Sonogashira C(sp3)–C(sp) coupling provides excellent complementary approaches to direct asymmetric C–C(sp3)/C(sp2) couplings.
More details of this work can be found here: “A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling” in Nature Chemistry.
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in