Energy efficient CO2 electrolysis to CO in acidic media
Published in Chemistry

CO2 electrolysis has the potential to substitute processes involving finite resources for the production of fuels and chemicals. On gold catalysts, CO2 reduction mainly yields CO (and hydrogen as by-product), which can for instance be used to compose syngas or as a building block in various chemical processes. To produce CO at large scale, currently gas diffusion electrodes (GDEs) are used. These are porous hydrophobic substrates in which catalyst nanoparticles are deposited (normally by air-brushing or spray coating) on one side. The electrolyte is fed on the catalyst side while CO2 is fed through the backside of the GDE, ensuring effective mass transport. The reaction happens at 3-phase boundaries formed by the catalyst particles, CO2 and electrolyte (shown in Figure 1). Although high current densities can be realized with these systems, up to now CO2 electrolysis to CO had been only carried out in neutral to alkaline media, and the energy efficiencies obtained were still not optimum. In our work, alternatively, we have performed the reaction in acidic media, and found that high selectivity and activity can be obtained in acidic electrolyte, using less electricity than if the same was done in neutral media.
This collaborative work started within the ELCoREL Marie Skłodowska-Curie Action Innovative Training Network. Fundamental work carried out at Leiden University showed that proton reduction, which is the main hydrogen evolution pathway taking place at low pH and low overpotentials, can be fully suppressed in acidic media. This happens, as long as the rate of CO/OH− formation from CO2 reduction is high enough to compensate the mass transfer of protons to the electrode surface.1 In another work, we also learned that alkali metal cations in the electrolyte (Li+, Na+, K+, Cs+) do not affect the proton reduction reaction (which is only a function of the proton concentration) while weakly hydrated cations like Cs+ lead to an exclusive enhancement in the CO2 reduction activity.2 At this point, we discussed the possibility of scaling up acidic CO2 electrolysis with our colleagues from Avantium, an innovation-drive company working in the emerging industry of renewable and sustainable chemistry. Matthew Philips, working at Avantium as PhD student, had the expertise to produce highly stable GDEs and to establish new CO2 reduction experimental setups.3 Combining and knowledge from both fundamental studies and the industry (and of course with the right tools), we carried out CO2 electrolysis at pH 2, 3 and 4 using 10 cm² GDEs, in both Li2SO4 and Cs2SO4 electrolytes. In Cs2SO4, we obtain CO faradaic efficiencies between 80-90%, operating at current densities up to 200 mA cm-2. In contrast, in Li2SO4 electrolyte, nearly no CO was formed at all current densities studied, which highlights the importance of the electrolyte identity for successfully carrying out CO2 electrolysis in acidic media. We consider it important that the conclusions from fundamental work carried out at Leiden University could be extended to a practical gas-diffusion electrode. However, the fundamental work could not predict how operating in acidic media would affect the process energy efficiency, which is very important as it basically dictates the process energy costs. Remarkably, by directly comparing our results in Cs2SO4 with results obtained using KHCO3 (neutral media), we find that operating in acidic media (all other system parameters were kept constant) lead to a 30 % improvement of the energy efficiency. We list in Figure 1 some advantages of working in acidic media over neutral/alkaline media.
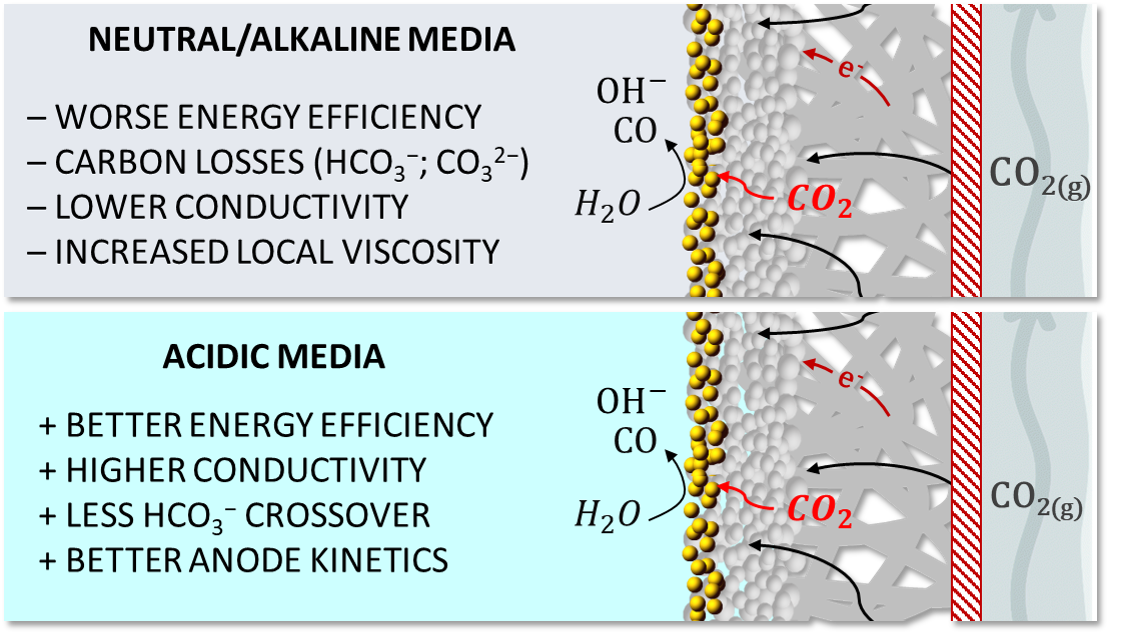
Figure 1. Advantages of carrying out CO2 electrolysis in acidic media, together with a schematic representation of the 3-phase boundary where CO2 reduction to CO takes place in gas diffusion electrode systems.
The main advance of the current work is showing that CO2 electrolysis to CO can be carried out in acidic media, at high current densities, with GDEs. Beyond that, another main result is that we find that the cell potential is significantly lowered by operating in acidic media, due to the superior conductivity of the acid electrolyte. This was concluded not only based on our work, but also when comparing with results from literature. We believe our work creates a new branch in the field of CO2 electrolysis and opens up numerous possibilities for optimizing the process further. It is also important to highlight the importance of bridging the knowledge between academia and the industry, in order to produce exciting science. I worked on fundamental electrocatalysis during most of my PhD, and only after performing the work at Avantium together with Matt I could clearly see (and understand) the bigger picture and where the future of CO2 electrolysis (roughly) lies.
Check out the publication via the following link: https://doi.org/10.1038/s41467-021-24936-6
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in