Evolutionary integration of fore- and hindlimb proportions within the bat wing membrane inhibits ecological adaptation compared to birds
Published in Ecology & Evolution and Zoology & Veterinary Science

How do animal traits evolve in relation to each other and how might this affect their capacity to adapt?
Birds and bats may provide the answer.
Animals are ensembles of evolving traits. Mechanical demands or limitations imposed by their growth and development might require traits to evolve in unison, despite the potential adaptive advantages that could be realized by the independent evolution of traits to optimize themselves to distinct functions. Limb evolution is a fitting example. Forelimb and hindlimb proportions tend to evolve cooperatively in many vertebrate lineages, perhaps because of their coordinated function, or their formation and growth under the influence of shared developmental processes.
However, many researchers have long held that birds and bats are exceptions, as evidenced by the spectacular divergences in form and function between their wings and legs. Indeed, divergent evolution between wings and legs is often cited as having had a key role facilitating ecological adaptation in birds and bats. Some researchers have argued that divergent wing and leg evolution may even have been integral to the initial acquisition of flight in these groups[1].
The study of birds and bats hence presents an opportunity to explore how the inter-related evolution of traits has shaped vertebrate evolutionary history.
Birds conform to expectations, but bats remain enigmatic:
Recent research, comparing skeletal proportions across a great diversity of bird species, has shown their wings and legs generally evolve independently of one another[2]. However, the evolutionary dynamics of bats still remain cloaked in mystery. Some studies comparing multiple individuals within the same species, have indicated that variation in the forelimb tends to occur independently of the hindlimb[3], but it is difficult to relate observations made at the microevolutionary scale to evolutionary divergences over geological eons. Indeed, variation between individual bats within the same species may be explained by ‘growing pains’[4], which are not inherited, and therefore do not contribute to long term evolutionary divergences.
Approach:
We aggregated measurements of wing and leg bone sizes from 111 species, representing 80% of bat families, including vampirish blood suckers (e.g. Desmodus rotundus), elegant nectar bats (e.g. Leptonycteris yerbabuenae), and insatiable insectivores (e.g. Pippistrellus pipistrellus). This scale of data collection, in conjunction with detailed descriptions of different bats' ecologies and existing family trees relating different bat species, allowed us to perform a study of similar scope to recent research in birds- exploring the inter-related evolution of traits, mapping ecological adaptation across body regions and reconstructing models of bats' exploration of different body traits over their evolutionary history.
Coauthor Prof. David Boerma reflects:
"Collecting the requisite data for this project was a multi-years process, interrupted by a global pandemic, and reignited by the promise of this study to reveal part of the fundamental dynamics at play for bat evolution. Countless nights, weekends, and holidays were spent in the bowels of the American Museum of Natural History, lovingly selecting specimens from among the jars of alcohol-preserved bats, bombarding them with high-power x-rays, and digitally reconstructing their skeletons from the resulting images. This study rewarded our patience, however, because the inclusion of over 100 species allowed us to draw more meaningful and convincing conclusions about the evolutionary dynamics of the flight skeleton in bats. And now, by depositing our scans and data in public repositories like Morphosource and GitHub, our labor can reach beyond the walls of our own labs. Making these data publicly available democratizes the scientific process so that other scientists who share our desire to disentangle the mysteries of bat flight evolution can carry the torch of inquiry in the direction of their own questions."
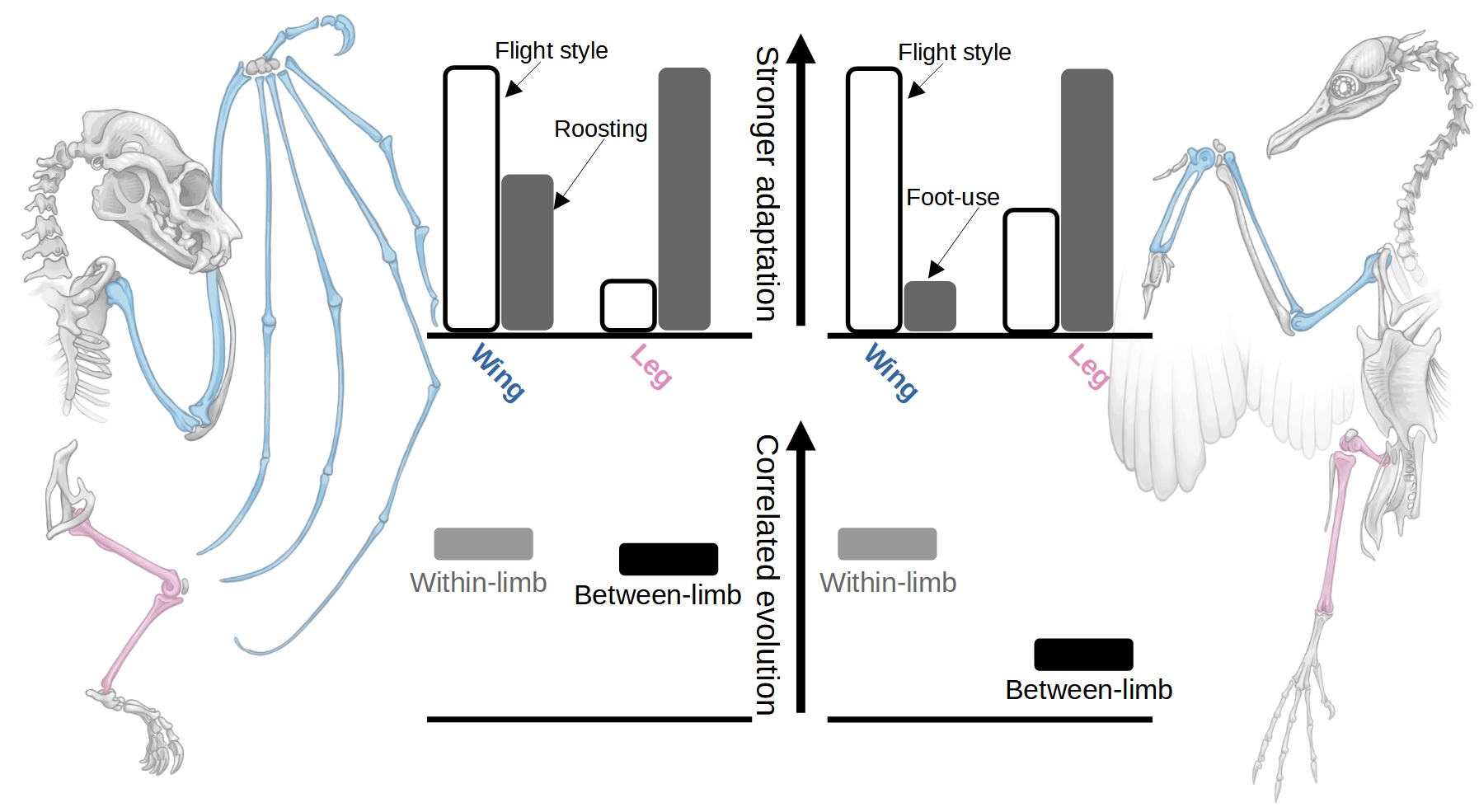
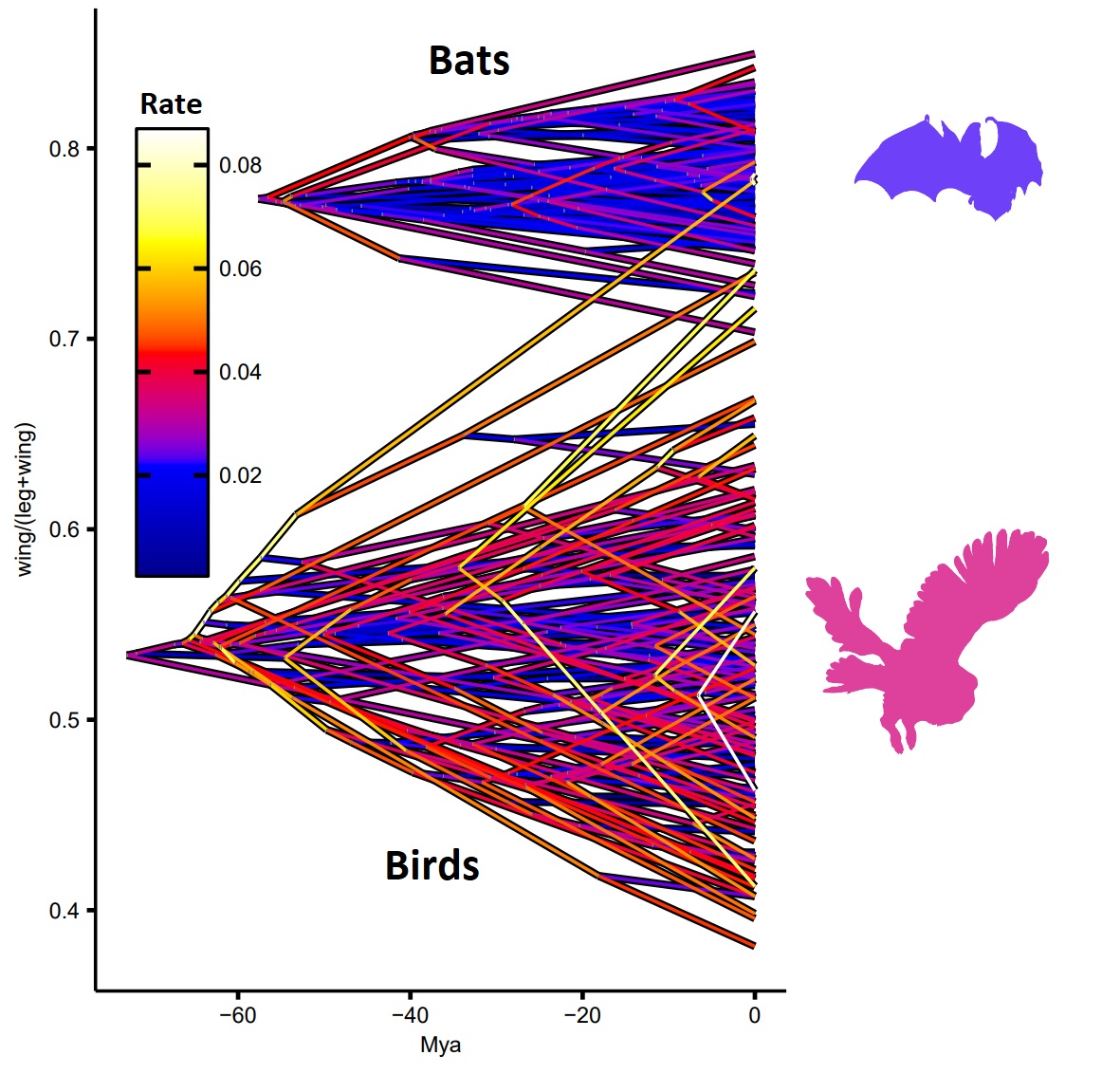
Results: Bat limb evolution is fundamentally different to birds, but why?
While adaptations towards different flight styles were largely confined to the bat wing (Figure 1), and adaptations towards roosting mostly concentrated in the leg and thumb (the portion of the wing used for climbing and scrambling), amazingly we found no evidence that bat wings and legs evolve independently of one another. This contrasts starkly with bird evolution, in which the adaptation of the wing towards flight style and the leg towards terrestrial, arboreal or aquatic ecologies, is actively mediated by the independent evolution of these structures (although substantial complexity exists across bird ecological adaptation of the skeleton[2]).
Principal Investigator Prof. Brandon Hedrick was surprised by this result:
“I first came at this project thinking that the work would help to establish a general rule for vertebrate flight. Given that the modular evolution of the wings and hindlimbs in birds has become something of a paradigm for explaining the diversification of birds, it seemed that bats must follow this same pattern. As we collected more data and started running preliminary analyses, it became clear that what was seemingly true in birds was not true in bats. Dr. Orkney began to refer to bats as having an integrated wing-suit rather than separate forelimb and hindlimb modules, an image that I think nicely captures how we can now begin to think about bat limb evolution. As always, evolution surprises me and being able to further probe this dataset with my students will undoubtedly reveal nuance and answers to questions we haven’t yet thought of.”
Bats' inability to adapt fore- and hindlimb independently may have major evolutionary consequences. Reconstructions of the ratio of wing length to leg length across birds and bats demonstrate that bird limb evolution has been much more dynamic than bat evolution. The majority of evolutionary variety across bats is cemented in a burst of innovation early in their history, before a period of relative stagnation (Figure 2). By contrast, birds have maintained high rates of evolutionary change throughout their history and have, furthermore, explored a wider repertoire of limb proportions.
Lead author Dr. Andrew Orkney suspects that bats' membranous wings prevent the independent evolution of their fore- and hindlimbs:
“The integrated evolution of bat limbs may be explained by their common development and function within a shared wing membrane, which extends along the entire side of the body. Crucially, the thumb is the sole component of the bat forelimb which partly protrudes outside this membrane (Figure 3). The evolution of the thumb length is substantially less associated with other limb bones and plays a key role in ecological adaptation. This implies that the membrane wing forces the correlated evolution of forelimb and hindlimb proportions in bats, inhibiting ecological adaptation,”
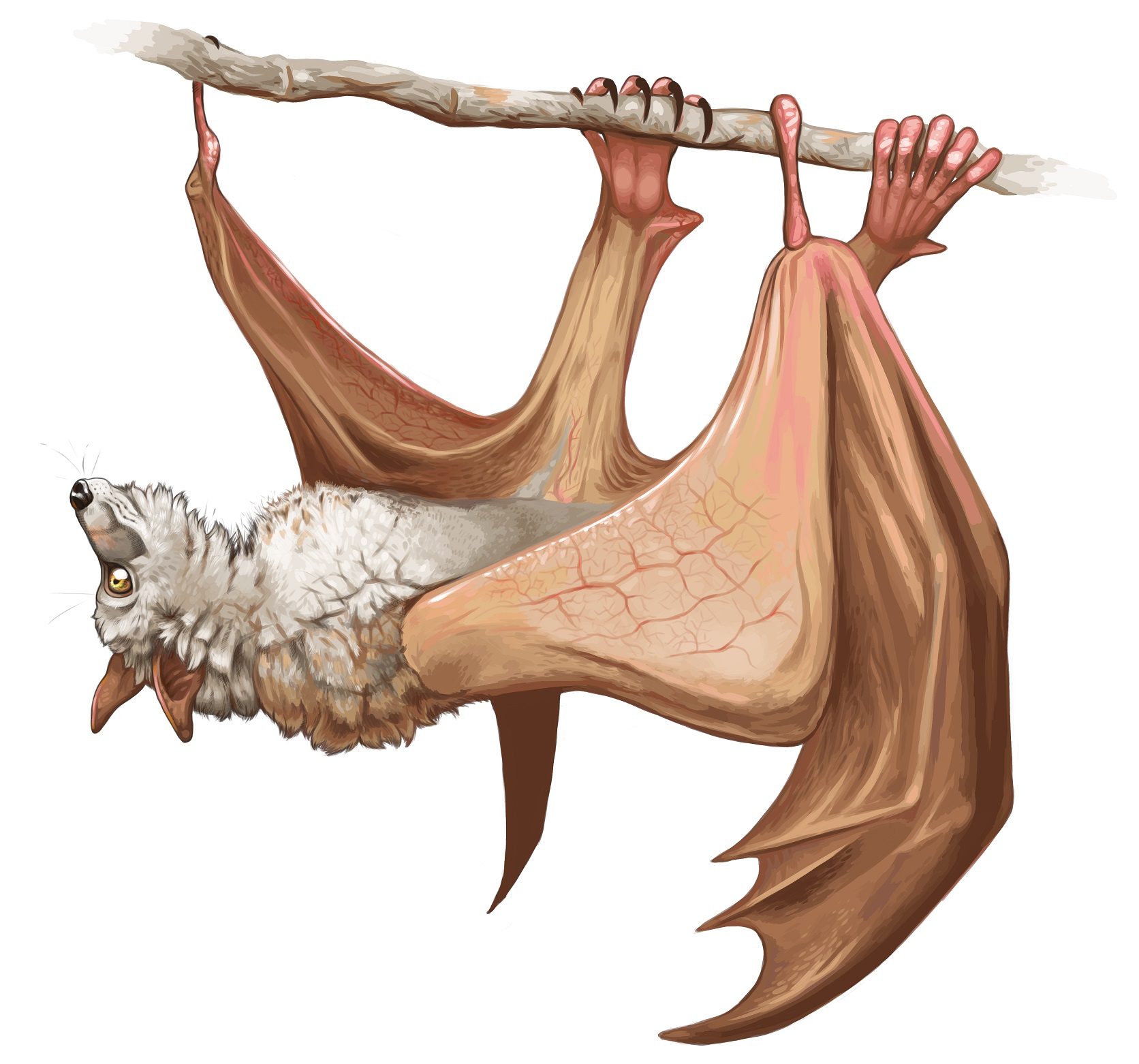
The sum of bat x-ray data and ecological descriptions compiled in this study will facilitate further new work engaging questions with wide-ranging scope in biology, such as the evolution of sexual differences and potential trade-offs between reproductive and ecological adaptation.
Caroline Goldstein, a post-baccalaureate who was not directly involved in the study, anticipates unlocking further secrets of bat evolution:
“These X-ray scans of bats representing a wide variety of species will allow me to investigate whether the pelvis of males and females is different across the order of bats. Baby bats weigh up to 43% the body weight of the adult mother at birth, imposing strict requirements on the pelvic shape of females. Flight, however, also imposes anatomical constraints, and as a result, bats must balance reproductive success with the anatomical requirements of flight.”
Further reading:
[1] Gatesy & Dial, 1996
Locomotor modules and the evolution of avian flight
[2] Orkney et al., 2021
[3] Young & Hallgrímsson, 2005
Serial homology and the evolution of mammalian limb covariation structure
[4] Adams, 1998
Evolutionary implications of developmental and functional integration in bat wings
Follow the Topic
-
Nature Ecology & Evolution

This journal is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biodiversity and ecosystem functioning of global peatlands
Publishing Model: Hybrid
Deadline: Jul 27, 2026
Understanding species redistributions under global climate change
Publishing Model: Hybrid
Deadline: Jun 30, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in