Fighting Superbugs with Microbial Chemistry: The Discovery of Coniontins Against Candida auris
Published in Biomedical Research
A Rising Threat from the Fungal World
When people think of infectious diseases, they usually picture bacteria or viruses. But in recent years, fungi, once a relatively quiet player in the infectious disease landscape, have emerged as a major global public health threat. At the forefront of this fungal rise is Candida auris.
First identified in 2009, C. auris is a multidrug-resistant yeast that can cause severe bloodstream infections, particularly in hospital and long-term care settings. What makes it especially concerning is its resistance to nearly all available antifungal drugs, its persistence on surfaces for weeks, and its high mortality rate in immunocompromised patients. The World Health Organization now lists C. auris as a critical priority pathogen.
So what happens when existing antifungal drugs are no longer effective? We turn to nature and chemistry for new solutions.
Nature’s Hidden Arsenal: Rediscovering Microbial Chemistry
Our research team has been exploring the natural chemical diversity of microbes in search of new antifungal compounds. Microorganisms have evolved to produce a wide range of bioactive natural products, many of which have inspired successful drugs like penicillin, erythromycin, and cyclosporin. However, since the “golden era” of antibiotic discovery, progress has slowed. One reason is that traditional screening methods often lead to the rediscovery of known compounds, buried within complex microbial mixtures.
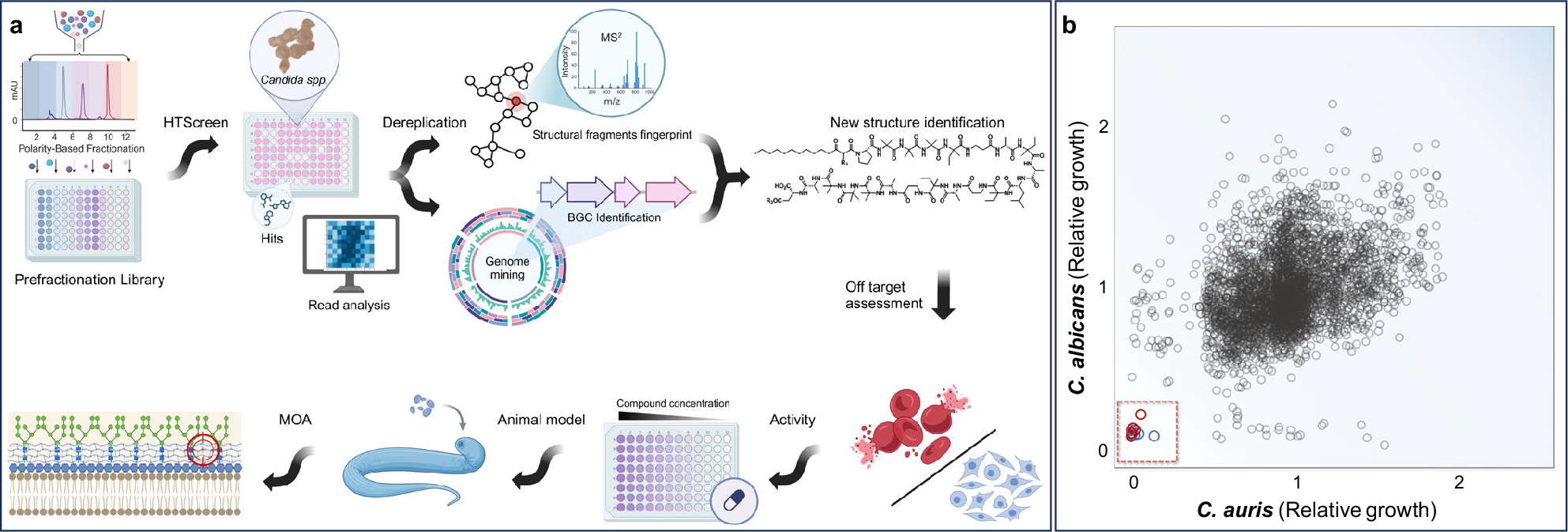
To overcome this bottleneck, we developed a prefractionation strategy combined with high-throughput screening, mass spectrometry, metabolomics, and computational dereplication (Figure 1). This allowed us to target overlooked or masked bioactive molecules. It was through this strategy that we discovered a previously unknown family of antifungal natural products—coniontins.
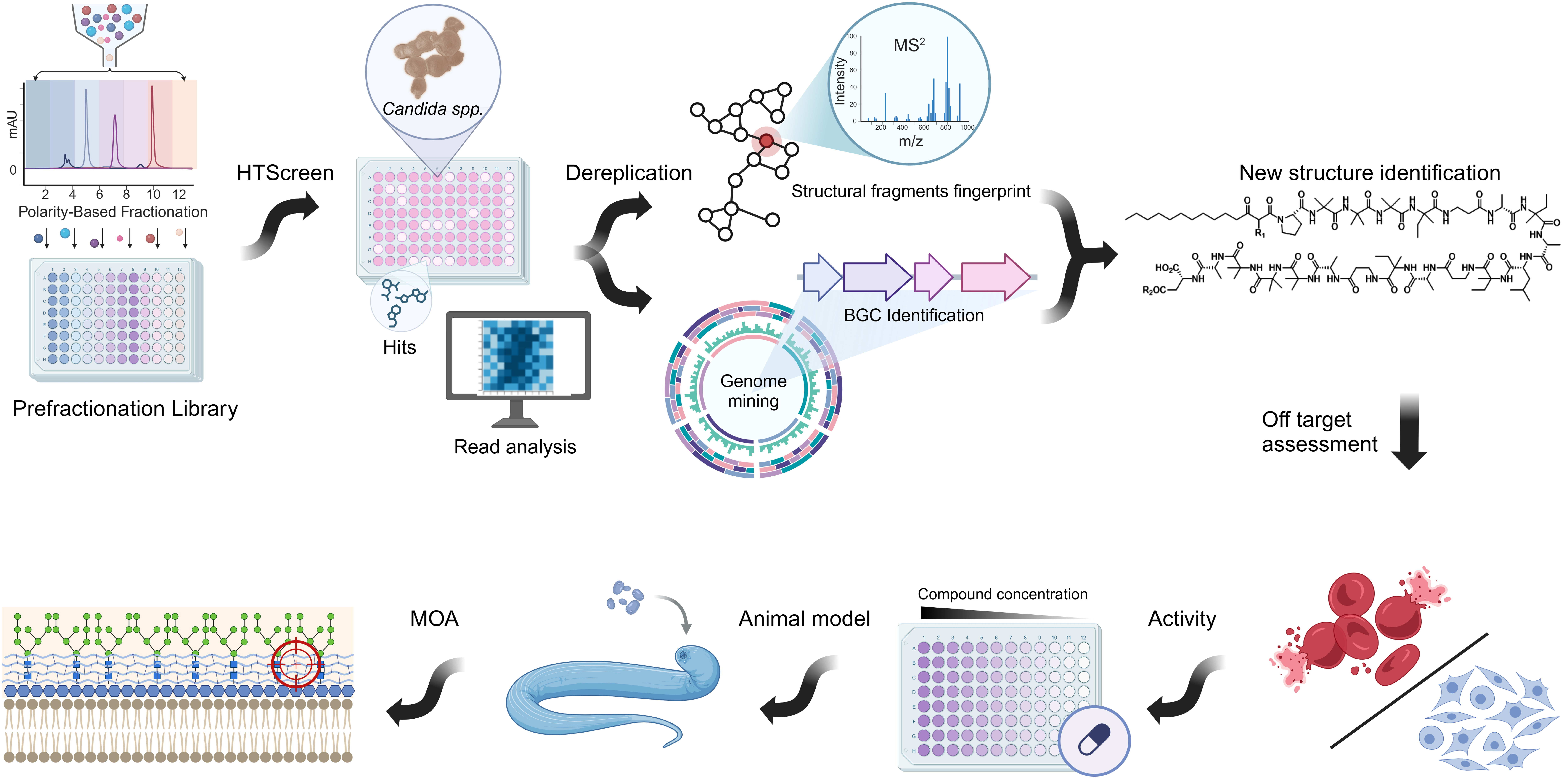
Figure 1: A scheme for the discovery of unexplored and derisked antifungal natural products
The Discovery: Uncovering Coniontins
While screening a prefractionated microbial extract library, we identified a fraction from the decomposition fungus Coniochaeta hoffmannii that showed potent, selective activity against C. auris.
Using a suite of analytical tools, including high-resolution mass spectrometry, NMR spectroscopy, and bioinformatics, we isolated and characterized a new group of molecules we named coniontins (Figure 2). These belong to the class of lipopetaibiotics: peptide-based natural products that feature a lipid tail attached to a 21-amino-acid peptide backbone rich in unusual, non-proteinogenic amino acids like α-aminoisobutyric acid (Aib). This distinctive structure gives coniontins a stable helical conformation and resistance to enzymatic degradation.

Coniontins demonstrated potent antifungal activity against a broad spectrum of multidrug-resistant C. auris clinical isolates, including strains resistant to all three major antifungal drug classes: azoles, echinocandins, and amphotericin B. Importantly, they exhibited low toxicity to mammalian cells in vitro and acted synergistically with caspofungin, a frontline clinical antifungal, highlighting their potential for use in combination therapy.
Listening to Molecules: How Do Coniontins Work?
Discovering a new antifungal agent is exciting but understanding how it works is essential.
To investigate the mechanism of action of coniontins, we treated fungal cells (C. auris, C. albicans, and Cryptococcus neoformans) with sublethal doses and examined changes in cell wall composition, cell morphology, and cell wall integrity. Our quantification and analysis revealed that these fungi activated a stress response characteristic of agents that target the cell wall. Specifically, we observed significant upregulation of chitin biosynthesis, a major component of the fungal cell wall that is tightly regulated.
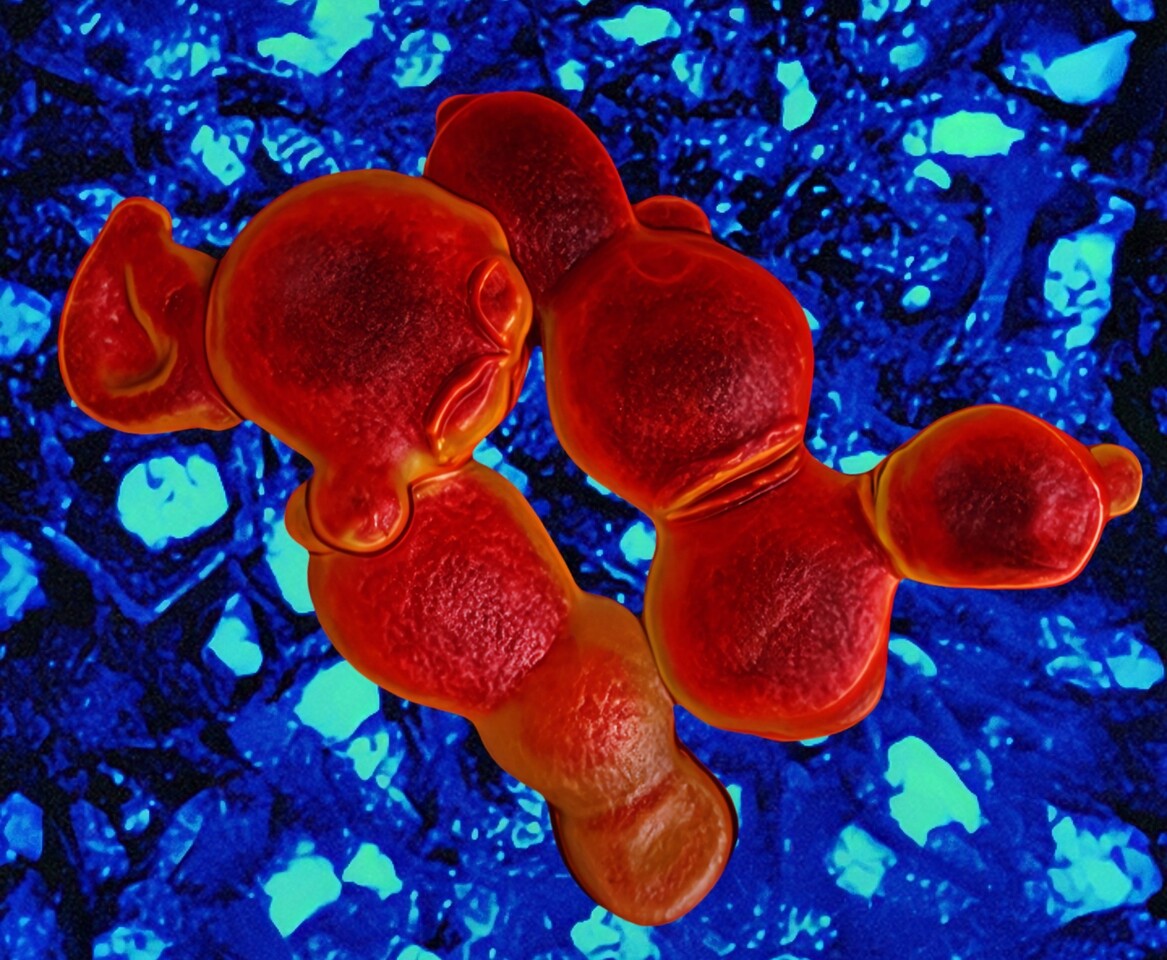
Using Alexa647-ConA staining, which labels the mannoprotein layer of the fungal cell wall, we visualized dramatic morphological changes after treatment. The fungal cells showed collapsed surfaces, abnormal budding with incomplete division, and wide necks connecting daughter cells. These phenotypes closely resembled those caused by echinocandins, which inhibit cell wall β-glucan synthesis (Figure 3).
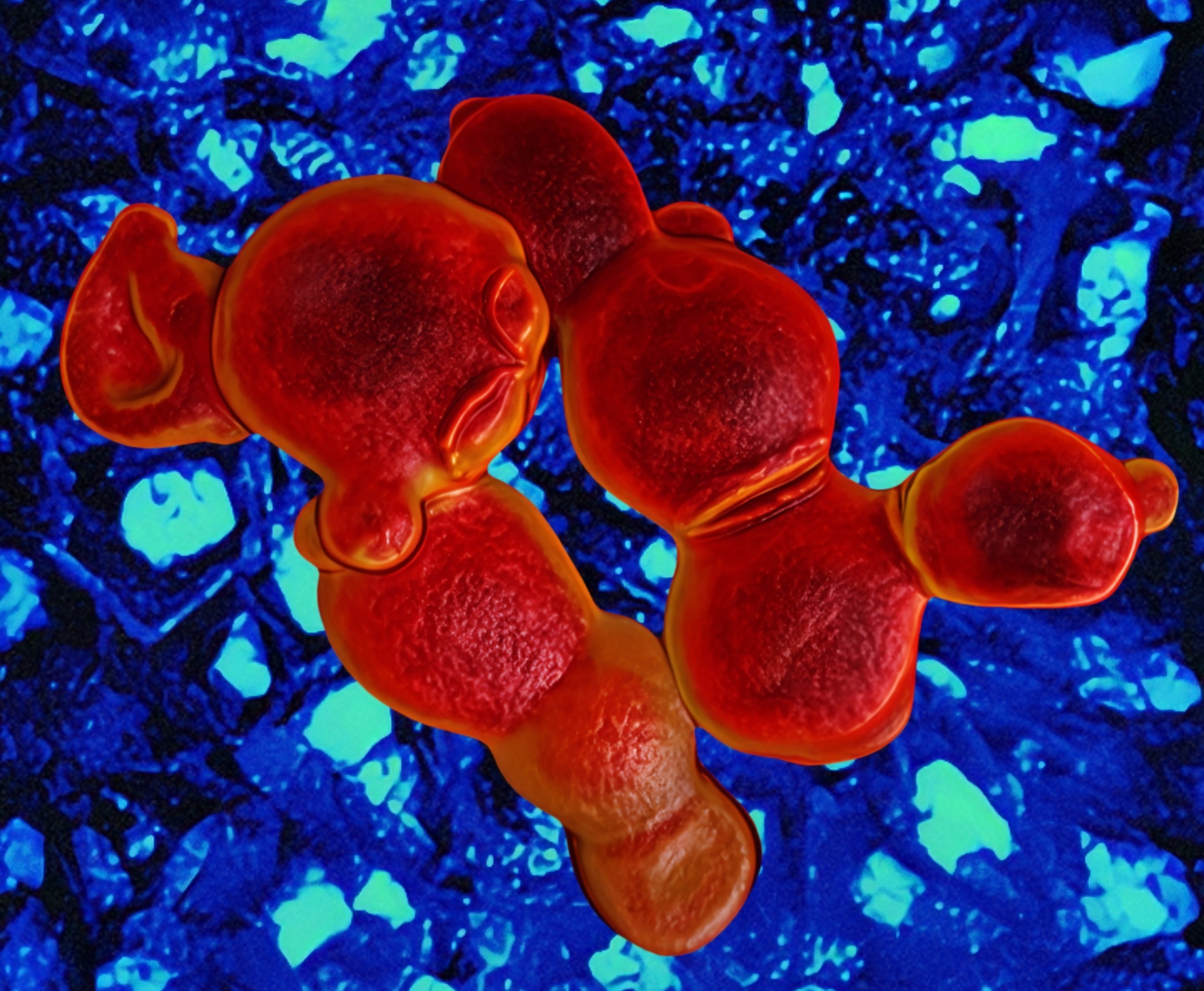
Further biochemical assays confirmed that coniontins bind to β-glucan in the fungal cell wall, compromising its integrity, causing thickening, swelling, rupture, and ultimately, cell death. Unlike many antifungals that disrupt intracellular targets and are susceptible to efflux or mutation-based resistance, coniontins act externally on the cell wall surface, making resistance less likely.
What’s more, in over 20 generations of lab-based exposure and a high-throughput screening across thousands of heterozygous yeast gene deletion mutants, we found no evidence of resistance against coniontins. This highlights their potential robustness in clinical settings.
Why It Matters
Coniontins are more than just a new class of antifungals, they represent a conceptual shift in fungal drug discovery.
For decades, most antifungal agents have targeted metabolic enzymes or intracellular pathways. But fungi are remarkably adept at adapting-mutating enzymes, overexpressing efflux pumps, and modifying metabolic routes. Coniontins bypass this arms race by targeting something less mutable: the fungal cell wall.
This brings us back to nature, but with a modern approach. By combining microbiology, synthetic biology, metabolomics, and machine learning, we are re-exploring nature’s chemical space and uncovering hidden gems like coniontins.
What's Next?
While coniontins are promising, our journey has only just begun. Key next steps include:
- Optimizing the compound’s pharmacokinetics and bioavailability for use in animal and human models
- Scaling up production using biosynthetic engineering or chemical synthesis
- Further investigating the synergistic activity with existing antifungals
- Expanding the structural diversity of coniontin analogs for broader-spectrum activity
We are eager to move coniotins along the development pathway. The next steps include producing it at scale through fermentation and formulating the new drug class so that it may eventually be suitable for intravenous (IV) delivery. Excitingly, we’ve already decoded the biosynthetic pathway in C. hoffmannii, which opens the door to scalable, engineered production (Figure 4). This not only enables practical drug development but also contributes to our understanding of how fungi naturally produce complex lipopetaibiotics.

Figure 4. Biosynthetic gene cluster schematic for coniontin production in fungi
A Broader Vision
The discovery of coniontins underscores a larger truth: microbial natural products remain a largely untapped reservoir of novel therapeutics. In the era of rising drug resistance, emerging pathogens, and global health crises, the solutions may still lie in the soil, in decaying wood, or even in fungal mycelia, waiting to be revealed.
By marrying classical natural product discovery with genomics, metabolomics, and computational tools, we can unlock nature’s full pharmaceutical potential and stay one step ahead of evolving pathogenic threats.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in