Filling the spectral gap of persistent luminescence
Published in Materials
As a promising self-sustaining light source that continuously emits light after the excitation source is removed, persistent luminescence (PersL) is gaining growing interest from scientists and industry professionals. The earliest descriptions of PersL can be traced back to ancient China, where historical records mention its use in night vision paintings and decorative objects. Despite this long history, scientific research on PersL materials remained scarce until the 1990s, when the discovery of the state-of-the-art PersL phosphor SrAl2O4:Eu2+/Dy3+ marked a pivotal moment in the field1. Since then, the development of PersL materials with bright and long-lasting emissions has progressed rapidly. These materials encompass a broad spectrum, ranging from organic compounds (e.g., small molecules, polymers, metal-organic complexes, and carbon dots) to inorganic crystals. The mechanisms behind PersL vary between these material types. In organic compounds, PersL is driven by radiative relaxation of electrons from the triplet state, which inherently has a long lifetime but is generally vulnerable in ambient conditions. In contrast, inorganic crystals rely on metastable trap states that gradually release trapped charge carriers to emitting centers, thereby sustaining luminescence over time. Nowadays, PersL materials have been popularly spotted as biomarkers for medical imaging2, signal indicators for emergency display3, as well as storage mediums for electromagnetic radiation, thermal field, and mechanical action4-5.
Nevertheless, a persistent challenge in the research community is the lack of methods for precise control over PersL spectrum within a single material system6. Currently, the primary strategy for tuning PersL color involves incorporating individual chromophores or luminescent centres into specific host compounds. This approach provides a facile way for designing emission profiles by deliberately selecting luminescent centres or their combinations (spectral mixing), which in turn results in discrete bands and spectral gaps in certain wavelength ranges, preventing the achievement of a full-spectrum PersL expression. This limitation poses significant hurdles to the development of advanced PersL materials with customizable emission properties.
This study presents an experimental investigation into PersL in single-phase Ca(Sr)ZnOS semiconductors. The pristine CaZnOS has been previously identified as a versatile platform for constructing multicolor PersL due to its diverse cation sites, which can accommodate various luminescent centres. Building on this foundation, we demonstrate that PersL tuning can also be achieved by modifying the host composition. Starting with the base compounds of CaZnOS and SrZnOS, which share the same crystalline structure, we obtain a series of continuous solid solutions with the chemical formula of Ca1-xSrxZnOS (x = 0–1). Structural characterizations confirm that the transition from CaZnOS to SrZnOS results in predictable changes in physical properties, thereby enabling controlled adjustment of PersL color.
Our approach for PersL tuning utilizes a donor-acceptor (D-A) pair activator composed of copper and yttrium, rather than a single activator. By doping these elements into the Ca(Sr)ZnOS lattice, we achieve highly controllable and linearly shifted PersL wavelengths by adjusting the Sr2+/Ca2+ ratio in the host materials. This method allows for precise tailoring of the PersL spectrum, enabling a smooth color transition from green to orange (Figure 1a and b). Remarkably, the crystals exhibit a high initial luminance of up to 5.36 cd m-2 and a maximum PersL duration of 6 hours. Unlike traditional methods that blend multiple emission peaks at different intensity ratios, our approach ensures a seamless spectrum coverage. The D-A pair activator thus offers exceptional flexibility in spectral tuning compared to traditional ionic luminescent centres, such as transition metal and lanthanide ions. This advancement makes full-color gamut expression of PersL a reality, significantly broadening the potential applications of these materials.
The idea of utilizing D-A pair as the activator to regulate PersL in Ca(Sr)ZnOS is inspired by the well-established ZnS phosphors7. D-A pairs are sub-bandgap defect levels that stem from impurity ions within the lattice. The electron-hole recombination from these D-A centres is a recognized mechanism, and has been exploited in various ion combinations such as Cu+-Al3+, Cu+-Cl-, and Ag+-Cl-. Wurtzite Ca(Sr)ZnOS as a derivative of ZnS is promising for tuning D-A emissions. While a single [ZnS4] tetrahedral site is available in ZnS, the Ca(Sr)ZnOS structure contains two types of cation sites: [ZnS3O] tetrahedron and [Ca(Sr)S3O3] octahedron. This structural arrangement provides larger spaces for impurity doping, permitting enhanced control over D-A characteristics and band structure of the host.
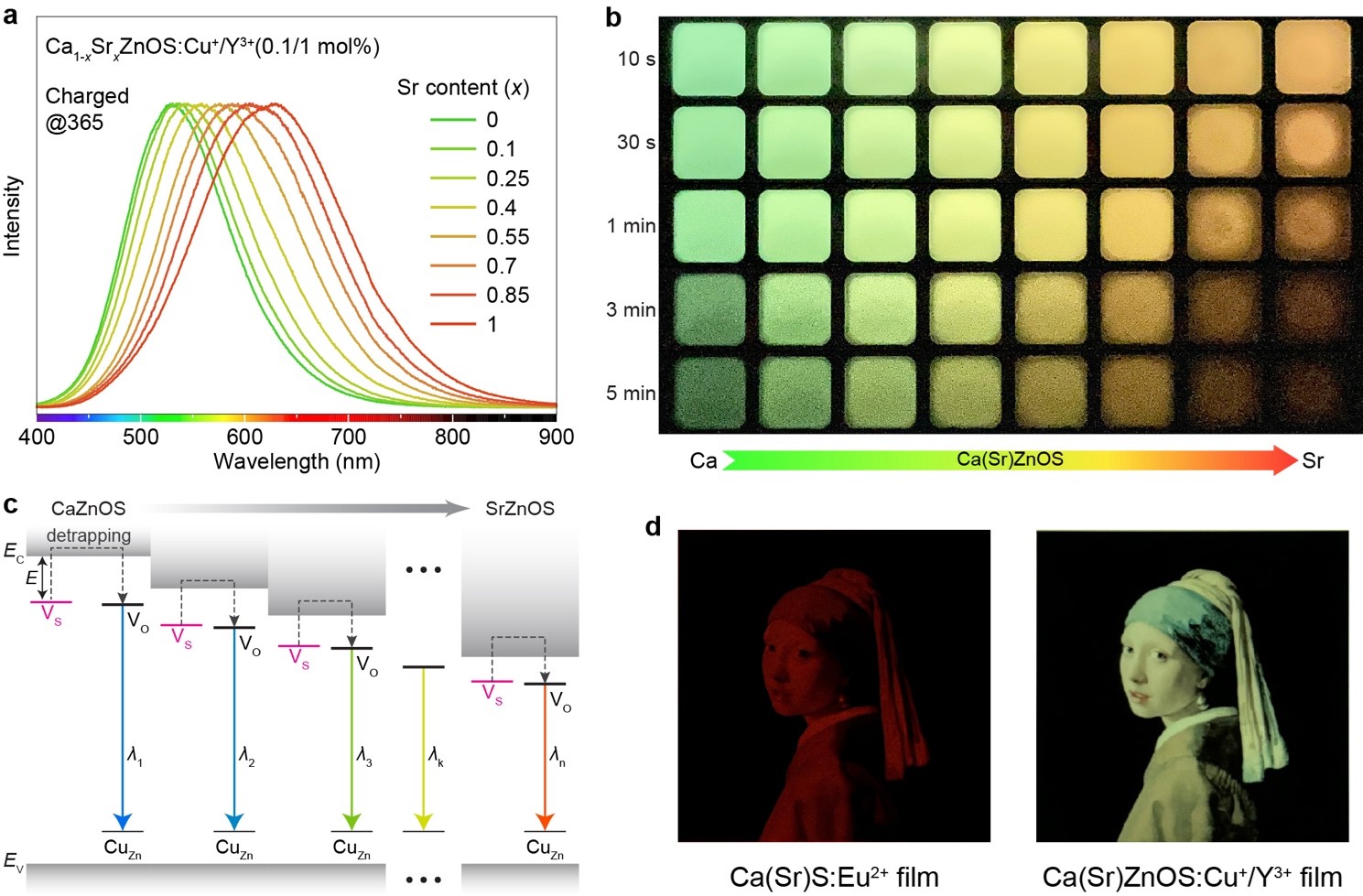
Figure 1. Continuous tuning of PersL Wavelength in Ca(Sr)ZnOS:Cu+/Y3+. a, PersL spectra of Ca1-xSrxZnOS:0.1%Cu+/1%Y3+ (x = 0−1) after turning off the UV charging light (4 W, 365 nm). b, PersL photographs of Ca(Sr)ZnOS:0.1%Cu+/1%Y3+ with various Sr contents. c, Band diagram illustration of the PersL mechanism. EC and EV refer to the energy of conduction band minimum and valence band maximum, respectively. d, Multicolor display through PersL film containing blended Ca(Sr)ZnOS:Cu+/Y3+ crystals in the presence of a patterned filter, which shows overwhelming color resolvability compared to the PersL film comprising conventional Ca(Sr)S:Eu2+.
Our research has led to several fundamental breakthroughs in PersL. Firstly, we establish a new strategy for controlling PersL by engineering the host band structure using constant D-A luminescent centers (Figure 1c). These developments enhance our understanding of PersL and pave the way for the rational design of novel PersL properties. Secondly, we achieve fine-tuning of PersL wavelength across a wide spectral range of over 100 nm, enabling on-demand design of PersL spectrum with a peak modulation precision of 5 nm. This level of PersL tuning within a single material system is unprecedented and could open new avenues for PersL research. Finally, Ca(Sr)ZnOS:Cu+/Y3+ crystals with finely tunable and bright PersL have been leveraged for multicolor display applications. The D-A activated Ca(Sr)ZnOS crystals exhibit superior color resolvability compared to conventional PersL emitters (Figure 1d).
In closing, the remarkable PersL tunability observed in Ca(Sr)ZnOS is poised to significantly broaden the range of applications for PersL materials. Particularly, using a single material system other than a mixture of materials for multicolor display could enhance device integrity and operational consistency by avoiding compatibility issues.
References
- Xu, J. & Tanabe, S. Persistent luminescence instead of phosphorescence: History, mechanism, and perspective. J. Lumin. 205, 581-620 (2019).
- Pei, P. et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011-1018 (2021).
- Chiatti, C., Fabiani, C. & Pisello, A. L. Long Persistent Luminescence: A Road Map Toward Promising Future Developments in Energy and Environmental Science. Annu. Rev. Mater. Res. 51, 409-433 (2021).
- Van der Heggen, D. et al. A Standalone, Battery-Free Light Dosimeter for Ultraviolet to Infrared Light. Adv. Funct. Mater. 32, 2109635 (2022).
- Petit, R. R., Michels, S. E., Feng, A. & Smet, P. F. Adding memory to pressure-sensitive phosphors. Light Sci. Appl. 8, 124 (2019).
- Gu, L. et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photonics 13, 406-411 (2019).
- Knowles, K. E. et al. Luminescent Colloidal Semiconductor Nanocrystals Containing Copper: Synthesis, Photophysics, and Applications. Chem. Rev. 116, 10820-10851 (2016).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in