Fluorescent Nitro-aromatics - An Endless Collaborative Challenge
Published in Chemistry

Since our team in Warsaw discovered tetraaryl-pyrrolo[3,2-b]pyrroles (TAPPs) in 2012, these molecules have been a constant source of surprises [1,2]. Their pyrrolopyrrolo cores are exceptionally electron-rich and exhibit strong electronic coupling with substituents located at positions 2 and 5. Through our search of strong two-photon absorbers with quadrupole acceptor-donor-acceptor architectures, in 2015 we synthesized a TAPP possessing two 4-nitrophenyl substituents at positions 2 and 5. Investigation by Prof. Aleks Rebane confirmed its strong two-photon absorptivity [3]. Notwithstanding the expected large two-photon absorption cross-section, this molecule served another curveball. During its purification, we observed that it exhibits exceptionally strong fluorescence but only in cyclohexane and other truly non-polar solvents. It took us by surprise since nitro-aromatics are known to be rather poorly fluorescent [4]. We quickly followed with the synthesis of other TAPPs with NO2 groups on the aromatic substituents at the critical positions 2 and 5 [5]. Nevertheless, placing the NO2 groups only at positions 4 versus the linkage with heterocyclic core gives rise to strong fluorescence.
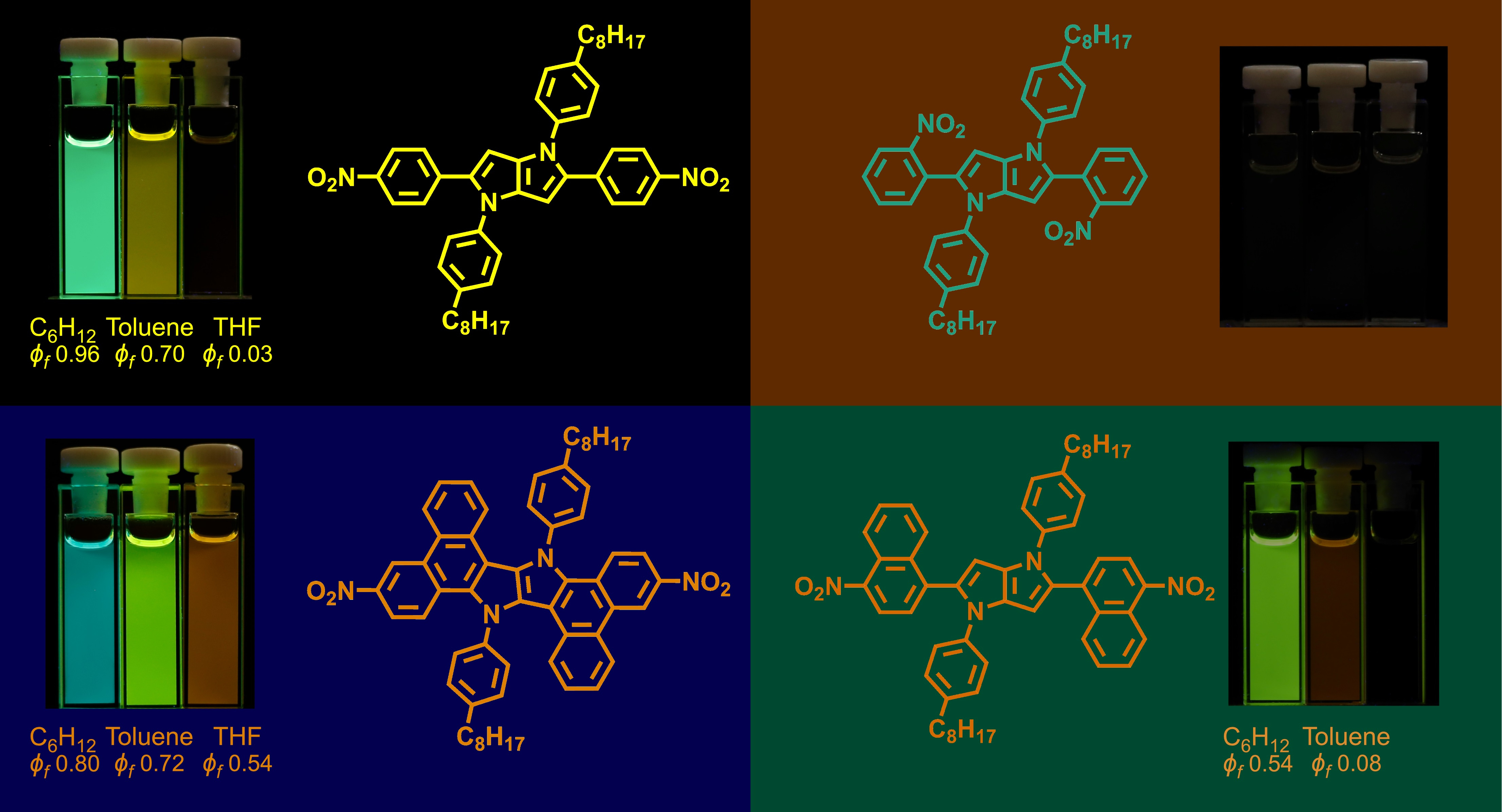
Repertoire of fluorescent and non-fluorescent bis-nitro-pyrrolo[3,2-b]pyrroles.
An intuitive explanation of this phenomenon did not present itself, and finding it became the dream of one of my most talented Research Associates – Dr. Yevgen Poronik. I have to admit I never before paid attention to fluorescence (or the lack of thereof) of nitro-aromatics. This particular perplexing problem has caught my interest in it on a broader scale, and we started synthesizing and studying various analogs of this nitroTAPP [6]. It was clear, however, that basic photophysical studies are not sufficient to solve the puzzle. Consequently, I turned to the good friend of mine Prof. Valentine I. Vullev from UC Riverside who incidentally was also interested in fluorescent nitroaromatics in pertinence to his charge-transfer (CT) research program [7], and agreed to study these bis-nitroTAPPs using time-resolved optical spectroscopy. These studies clarified that internal conversion, rather than triplet formation, was principally responsible for the quenching of the fluorescence for polar media or when the NO2 groups were not at positions 4. The solvent trends also revealed that intramolecular CT dominate the excited-state dynamics of these compounds. Nevertheless, some key details still remained unsolved. For example, why 2-nitrophenyl substituents quench fluorescence while 4-nitrophenyls do not, and moreover what is the exact mechanism by which polar solvents kill the emission even of the latter ones. While the project was on hold for about 1.5 years, our interest in fluorescence of nitro-aromatics grew and gave new fruits [8]. In parallel it induced my interest in excited-state symmetry breaking of TAPPs which led to amazing collaborations with Prof. Eric Vauthey [9] and with Prof. Denis Jacquemin [5,6,8].
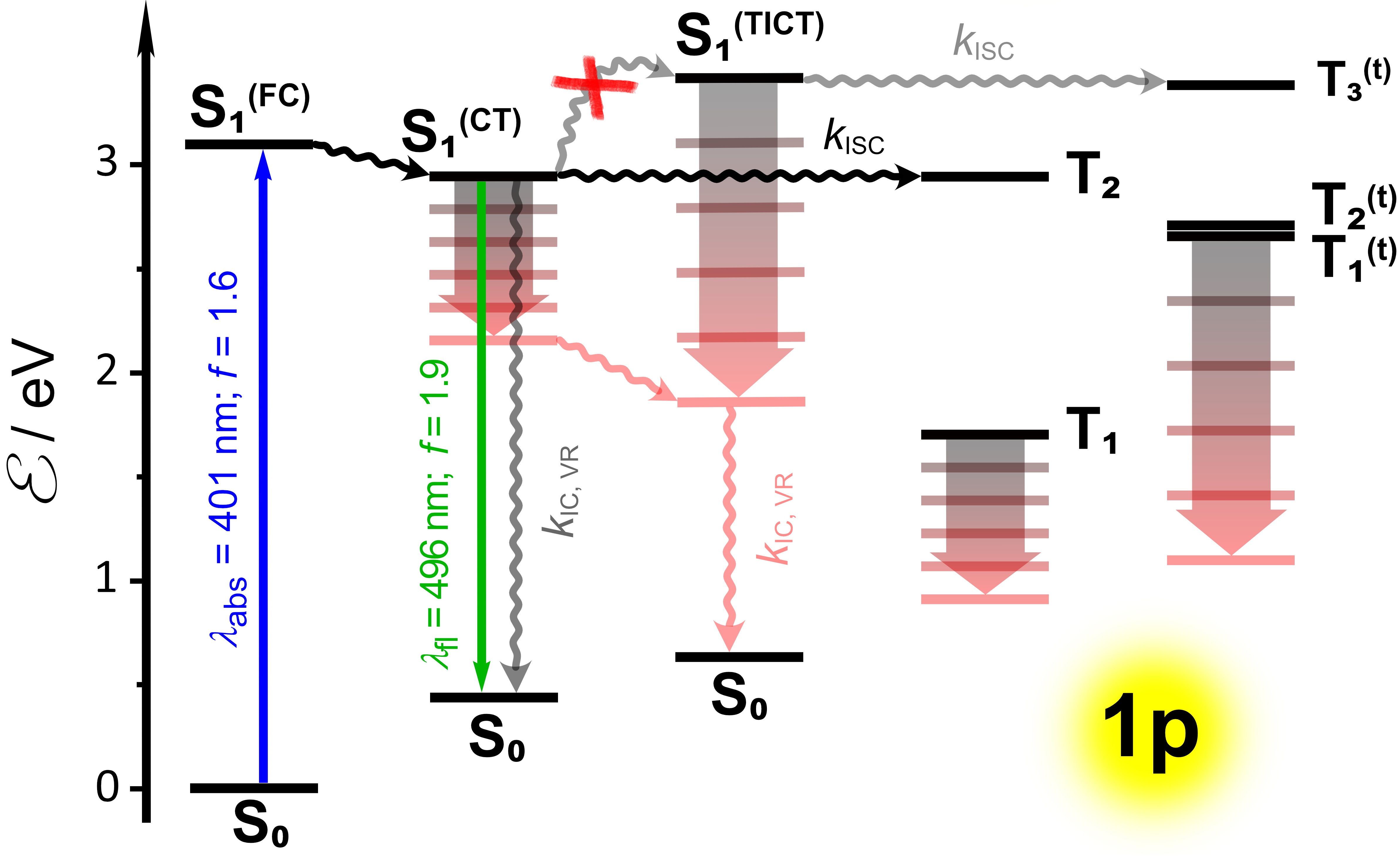
Simplified Jablonski diagram for 2,5-bis(4-nitrophenyl)pyrrolo[3,2-b]pyrrole.
In the search of the missing pieces of the puzzle, we approach a renowned theoretical chemist Prof. Hans Ågren from Sweden. He became truly interested in this project, and together with Dr. Glib Baryshnikov, studied computationally the bis-nitroTAPPs. Things began to look clear. The combined results from time-resolved spectroscopy and computational interrogation allowed us to put together the puzzle. Our studies reveal the crucial importance of the molecular geometry of the nitroaromatics for making them fluoresce. Planarity of the structures ensures a spatial overlap between the orbitals carrying the positive charge of the oxidized donor (pyrrolopyrrolo core) and the negative charge of the reduced acceptor (nitrophenyl substituent) even for polarized CT states. Such orbital overlap, indeed, translates to large radiative-decay rates and strong fluorescence, while the CT character of the excited states reduces the propensity for intersystem crossing (ISC) leading to triplet formation. Torsional degrees of freedom allow conformations with orthogonality between the rings of the donor and the acceptor breaking the delocalization of the frontier orbitals and diminishing orbital overlap. Therefore, such twisted intramolecular charge-transfer (TICT) excited state are dark. Furthermore, the orthogonal geometry of the TICT states enhances ISC. Solvent polarity stabilizes such TICT states with relatively well separated charges localized on the donor and the acceptor. The steric hindrance between the NO2 groups and the pyrrolopyrrole maintains orthogonality in the 2-nitrophenyl TAPPs, which is consistent with the lack of detectable fluorescence and the sub-picosecond excited-state lifetimes. These paradigms bring us closer to electron-deficient nitroaromatics that, in addition to their characteristics as n-type conjugates, also have attractive optical properties.
References:
1 A. Janiga, E. Glodkowska-Mrowka, T. Stoklosa and D. T. Gryko, Asian J. Org. Chem. 2, 411-415 (2013).
2 M. Krzeszewski, D. Gryko and D. T. Gryko, Acc. Chem. Res. 50, 2334−2345 (2017).
3 D. H. Friese, A. Mikhaylov, M. Krzeszewski, Y. M. Poronik, A. Rebane, K. Ruud and D. T. Gryko, Chem. Eur. J. 21, 18364-18374 (2015).
4 M.-C. Chen, D.‐G. Chen and P.‐T. Chou, ChemPlusChem https://doi.org/10.1002/cplu.202000592 (2020).
5 Ł. G. Łukasiewicz, H. G. Ryu, A. Mikhaylov, C. Azarias, M. Banasiewicz, B. Kozankiewicz, K. H. Ahn, D. Jacquemin, A. Rebane and D. T. Gryko, Chem. Asian J. 12, 1736-1748 (2017).
6 Ł. G. Łukasiewicz, M. Rammo, C. Stark, M. Krzeszewski, D. Jacquemin, A. Rebane and D. T. Gryko, ChemPhotoChem 4, 508-519 (2020).
7 E. M. Espinoza, B. Xia, N. Darabedian, J. M. Larsen, V. Nuñez, D. Bao, J. T. Mac, F. Botero, M. Wurch, F. Zhou and V. I. Vullev, Eur. J. Org. Chem. 343-356 (2016).
8 K. Skonieczny, I. Papadopoulos, D. Thiel, K. Gutkowski, P. Haines, P. M. McCosker, A. D. Laurent, P. A. Keller, T. Clark, D. Jacquemin, D. M. Guldi and D. T. Gryko, Angew. Chem. Int. Ed. 59, 16104-16113 (2020).
9 Z. Szakacs, M. Tasior D. T. Gryko and E. Vauthey, ChemPhysChem 21, 1718-1730 (2020).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in