Forget the chicken or the egg: It doesn’t matter if RNA or proteins came first, rather that they need each other
Published in Chemistry

How did life begin? - is one of the most intriguing questions in all science. How did cooperative, interdependent relationships between nucleic acids and proteins arise? RNA makes protein in the ribosome and protein makes RNA in polymerases. This interdependence of biology leads to apparent paradoxes for the start of life: which came first, nucleic acids or proteins – the chicken or the egg?
Many researchers favor the RNA world hypothesis, in which RNA came first and initially served dual roles as both a genetic polymer and as an enzyme. According to this theory, RNA once self-replicated, but has now lost that function in biological systems. This model is attractive because avoids the extreme improbability of simultaneous independent origins of two different types of polymers. Over time the RNA World incrementally invented the ribosome, giving rise to the current biological system comprised of RNA, DNA, and protein.
In an alternative model, the evolution of nucleic acids and proteins was concerted. Multiple types of polymers were intimately connected from the very beginning (i.e. a Ribonucleoprotein World). In this model, the extreme improbability of simultaneous origins of two different types of polymers is solved by assuming their origins were not independent, but were linked. Our recent publication in Nature Communications provides experimental support for this model. The manuscript describes specific mechanisms of chemical linkage that could have operated during the origins of biopolymers. The results suggest that neither nucleic acids nor proteins came first, but rather that RNA and proteins were selected together through a process of co-evolution. Mutualistic relationships between molecules were important from the very beginnings of biology.
Our study was supported by the National Science Foundation (NSF) and NASA under the auspices of the Center for Chemical Evolution (CCE). In the CCE, we study how prebiotic molecules could have formed and functioned in the era preceding life on Earth. We might never know exactly how chemical evolution led to emergence of complex living systems, but as chemists we can explore the underlying principles that likely governed the era of chemical evolution. We investigate functional polymers and molecular interactions that might have existed on the Hadean Earth about 4 billion years ago. In an unbounded and agnostic approach, we look for plausible prebiotic proto-polymers that could have been ancestors to today’s biopolymers, harboring more primitive structures and functions.
Polyesters and depsipeptides, which are structurally similar to peptides but contain backbone ester linkages in place of some or all amide bonds, have been proposed as the chemical ancestors of present-day proteins. In a recent publication in PNAS (2019) we investigated how positively charged depsipeptides could have formed on the prebiotic earth. Given that electrostatics are key elements of protein-nucleic acid interactions in extant life, we hypothesized that these cationic proto-peptides might engage in beneficial interactions with nucleic acids.
When we started this project back in 2017, we were very happy to provide a connection between two of the core teams in the CCE, the Proto-Peptides team and the Proto-Nucleic Acids team, and look for cooperation between the two types of polymers at the molecular level. If peptides and nucleic acids co-evolved during the origin of life, then early peptides and oligonucleotides almost certainly interacted in functionally productive ways that led to their mutual chemical selection. In this latest publication (Nature Communications, 2020) we show that cationic proto-peptides, either produced as heterogenous mixtures from plausibly prebiotic dry-down reactions or synthetically prepared in pure form, can engage in direct, mutually-stabilizing interactions with RNA. Cationic proto-peptides significantly increase the thermal stability of folded RNA structures. We were very pleased that even very short cationic depsipeptides, such as those produced as mixtures in our model dry-down reactions, were able to stabilize RNA. In turn, we found that RNA reduces the rate of hydrolysis of depsipeptide ester bonds by more than 30-fold. We were quite surprised to see this dramatic result, which supports the importance of mutualistic molecular interactions for increasing persistence of oligomers over time. If the oligomers that are better able to fold or form molecular assemblies hydrolyze slower than the less assembly-competent oligomers, then there would be a natural enrichment over time of the population of oligomers that are capable of folding and assembly. Our results help provide experimental support for a mechanism for the selective buildup and enrichment of a subset of molecules out of a messy prebiotic mixture.
Moreover, one of the long-standing questions in origins-of-life research centers on how the proteinaceous side chains and the protein backbone were selected during the earliest phases of evolution. Our work suggests a possible mechanism for this selection - we show that proto-peptides containing proteinaceous amino acids (i.e. amino acids that are incorporated into proteins during translation) adjacent to ester bonds generally promoted RNA duplex thermal stability to a greater magnitude than did analogous sequences containing non-proteinaceous residues. This is a purely chemical driving force that could have led to the selection of certain amino acids over others. This selection was not random, and we now better understand its origins.
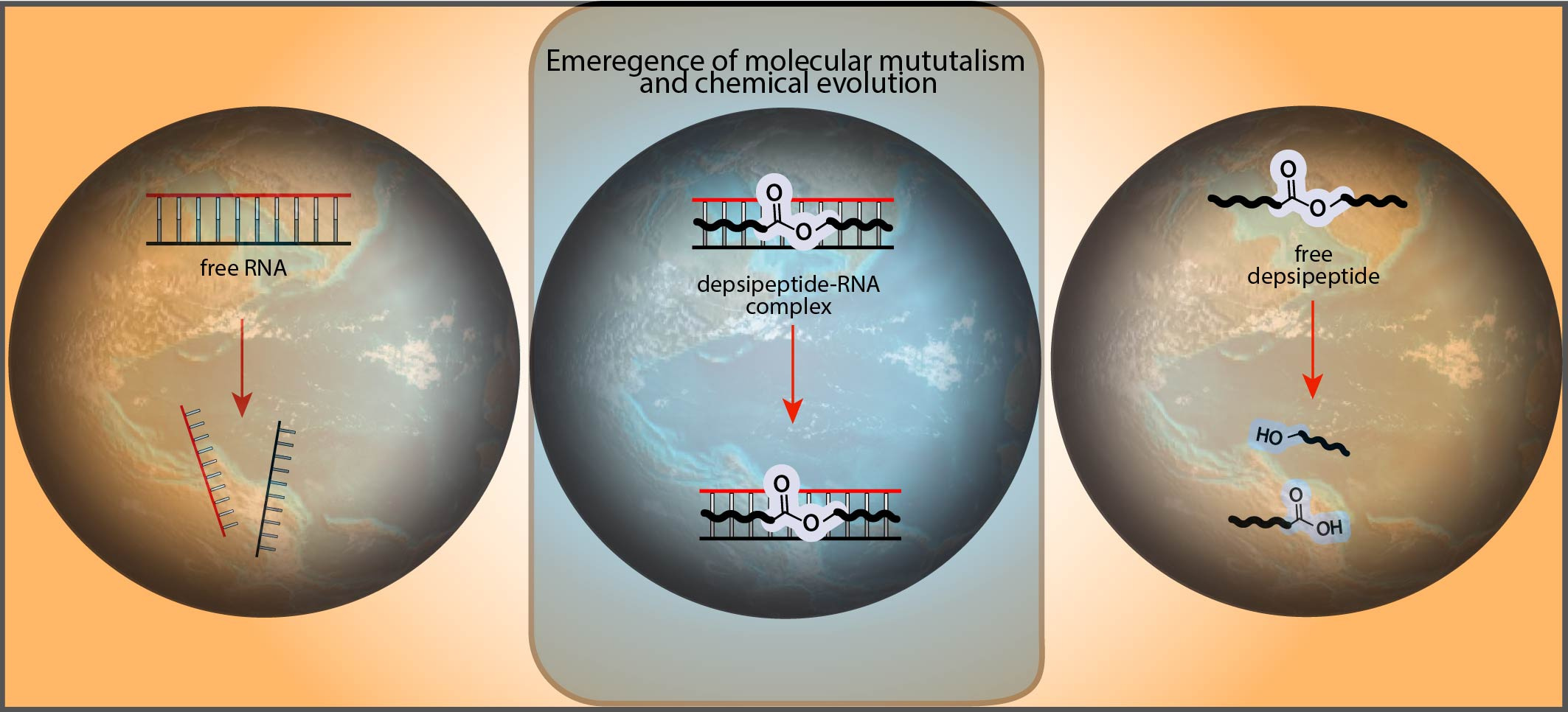
Schematic illustration of molecular cooperation between proto-peptides and RNA that could have fostered their co-evolution.
To the best of our knowledge, this report is the first experimental demonstration of chemical mutualism involving RNA and proto-peptides. The results of our study support the idea that the functional mutualism between RNA and peptides in extant biology has ancient origins and reflects a long co-evolutionary history.
The study was supported by the National Science Foundation and the NASA Astrobiology Program under the NSF Center for Chemical Evolution (grant CHE-1504217).
References:
- Frenkel-Pinter M, Haynes JW, Mohyeldin AM, C M, Sargon AB, Petrov AS, Krishnamurthy R, Hud NV, Williams LD* and Leman LJ*. (2020) Mutually Beneficial Interactions Between Proto-Peptides and RNA. Nat Comm. https://doi.org/10.1038/s41467-020-16891-5
- Frenkel-Pinter M, Haynes JW, C M, Petrov AS, Burcar BT, Krishnamurthy R, Hud NV, Leman LJ* and Williams LD*. (2019) Selective Incorporation of Proteinaceous over Non-Proteinaceous Cationic Amino Acids in Model Prebiotic Oligomerization Reactions. Proc Natl Acad Sci U S A. 116(33):16338-16346.
- Bowman JC, Petrov AS, Frenkel-Pinter M, Penev PI and Williams LD. (2020) The Root of the Tree: Significance, Evolution and Origins of the Ribosome. Chem Rev. doi: 10.1021/acs.chemrev.9b00742
- Frenkel-Pinter M, Samanta M, Ashkenasy G* and Leman LJ*. (2020) Prebiotic Peptides: Molecular Hubs in the Origin of Life. Chem Rev. doi: 10.1021/acs.chemrev.9b00664
- Forsythe JG, Yu S.-S., Mamajanov I, Grover MA, Krishnamurthy R, Fernandez FM, Hud NV. (2015) Ester-mediated amide bond formation driven by wet-dry cycles: a possible path to polypeptides on the prebiotic earth. Angew Chem Int Ed Engl 54(34):9871-9875
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in