From development to tumorigenesis: novel human brain organoids
Published in Cancer, Protocols & Methods, and Cell & Molecular Biology

Size, cell types and morphology are only a few of the features that make the human brain unique from other species (ref. 1). Due to its complexity, these characteristics have hampered the modelling of the human brain in a dish, making it more difficult for scientists to understand brain development and brain-related diseases.
Decades of research has resulted in the development of innovative models to mimic complex organ structures in vitro, to understand how organs develop but also to investigate what goes awry in disease. Our brain underwent major evolutionary changes, resulting in the value of human-based models to study features specific to the human brain. So far, a frequently chosen model relies on the generation of 3D structures resembling the brain by differentiating pluripotent stem cells using specific chemical cocktails (so-called PSC-derived organoids). These models were fundamental to discover previously unknown brain development mechanisms that are typical of humans (ref. 2).
With our lab’s background in working with organoid models also derived from other organs, such as the liver (ref. 3), which are instead directly derived from tissue (so-called TSC-derived organoids), we wondered: why do tissue-derived brain organoids not exist yet? Up until now, small stem cell cultures, termed neurospheres, could be derived from tissue, but these structures lack cellular diversity and structural complexity. We thus set out to identify the conditions to expand the human fetal brain in culture as organoids. Given the emerging role of the extracellular matrix present in the developmental brain in supporting the proliferation of neural progenitor cells, this suggested us that starting with tissue fragments, in which a higher tissue structure is maintained, could physiologically support a tissue-like expansion. We therefore tried to culture small fragments of fetal brain in a simplistic culture medium where the gross tissue structure (germinal zones) as well as native ECM are maintained. To our surprise, the tissue structures grew into organized structures and expanded in culture! We termed these Fetal-derived Brain Organoids (FeBOs) (ref. 4). The FeBOs represent a “snapshot” of the tissue of origin and this characteristic makes them a unique model to study development.
In our latest protocol (ref. 5), we provide a comprehensive overview of the establishment and culturing of FeBOs as well as different methodologies that can be used for downstream applications such as performing compound screening. The organoids can be maintained long-term in an expansion state that allows us to grow and split them into smaller organoids that self-assemble again into new organoids, maintaining the same cellular and architectural complexity. This expansion characteristic makes these brain organoids unique and allows us to use them for a myriad of applications!
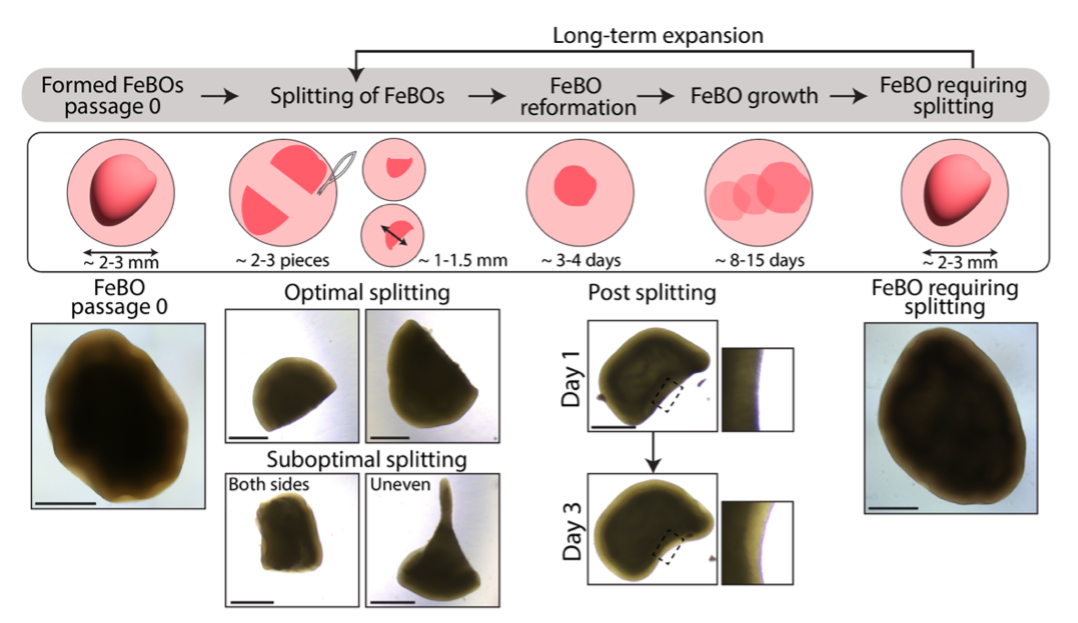 FeBOs grow overtime in culture and can be split to allow the formation of new organoids from individual FeBO fragments, thus allowing to study aspects of brain expansion.
FeBOs grow overtime in culture and can be split to allow the formation of new organoids from individual FeBO fragments, thus allowing to study aspects of brain expansion.
The expansion capability displayed by FeBOs is not only interesting when studying development, but it can also be exploited in the context of tumor modelling. For this, we combined the state-of-the-art of the CRISPR-Cas9 editing system with our FeBO cultures to precisely introduce tumorigenic mutations in sparse cells in the FeBOs. Once the mutations were introduced, mutant cells can gain an enhanced proliferation capacity, a typical characteristic of tumor cells, allowing them to overgrow the non-mutant, wild-type, cells in a relatively short time. This method, which we extensively describe in this protocol, enables the characterization of brain tumor initiation, opening new avenues for further investigations on the putative cell of origin in different tumors and on the type of mutations needed for neural cells to become tumorigenic. As of now, we have tested several common mutations known to play a role in glioblastoma, but different combinations could be further tested to mimic many different tumor (sub)types and also across different brain regions.
Once mutant cells fully overgrow the wild-type cells in the organoids, it is possible to establish mutant FeBO lines which can be used for higher throughput downstream analyses, with little organoid-to-organoid variation. A different scenario can be observed when introducing less potent tumor mutations, which often “struggle” to overtake the healthy population, showing how different genetic backgrounds of a tumor impact the tumor progression in healthy tissue, ultimately allowing us to learn about the needs and potential vulnerabilities of brain tumors.
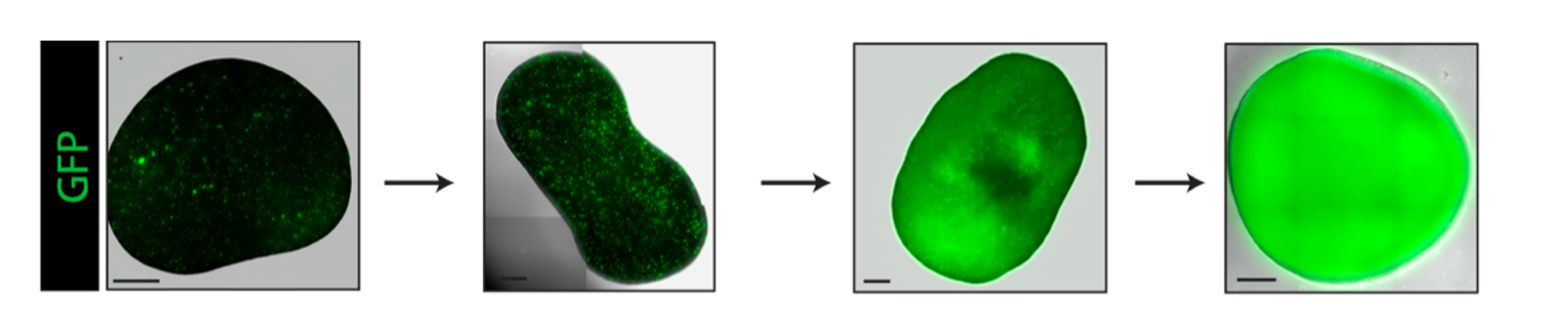 Example of mutant cells (in green, GFP+) that can overtake the whole healthy FeBO tissue over time resulting in the generation of a fully mutant FeBO.
Example of mutant cells (in green, GFP+) that can overtake the whole healthy FeBO tissue over time resulting in the generation of a fully mutant FeBO.
What are the future possibilities of FeBO cultures? We can foresee the model’s usefulness for studying many different biological questions, ranging from several facets of brain development, brain tumor biology, as well as infectious diseases and possibly even the testing of novel therapeutics. With this published protocol, we hope to have facilitated the ability of researchers to widely adopt and further adapt this model, broadening its utility and advancing the frontiers of neuroscience and medical research.
References
- Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M. & Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 176, 139–148 (2016).
- Chiaradia, I. & Lancaster, M. A. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Neurosci. 23, 1496–1508 (2020).
- Hendriks, D., Artegiani, B., Hu, H., Chuva de Sousa Lopes, S. & Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Protoc. 16, 182–217 (2021).
- Hendriks, D. et al. Human fetal brain self-organizes into long-term expanding organoids. Cell 187, 712-732.e38 (2024).
- Pagliaro, A., Andreatta, F., Finger, R., Artegiani, B. & Hendriks, D. Generation of human fetal brain organoids and their CRISPR-engineering for brain tumor modelling. Protoc. https://doi.org/10.1038/s41596-024-01107-7(2025).
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in